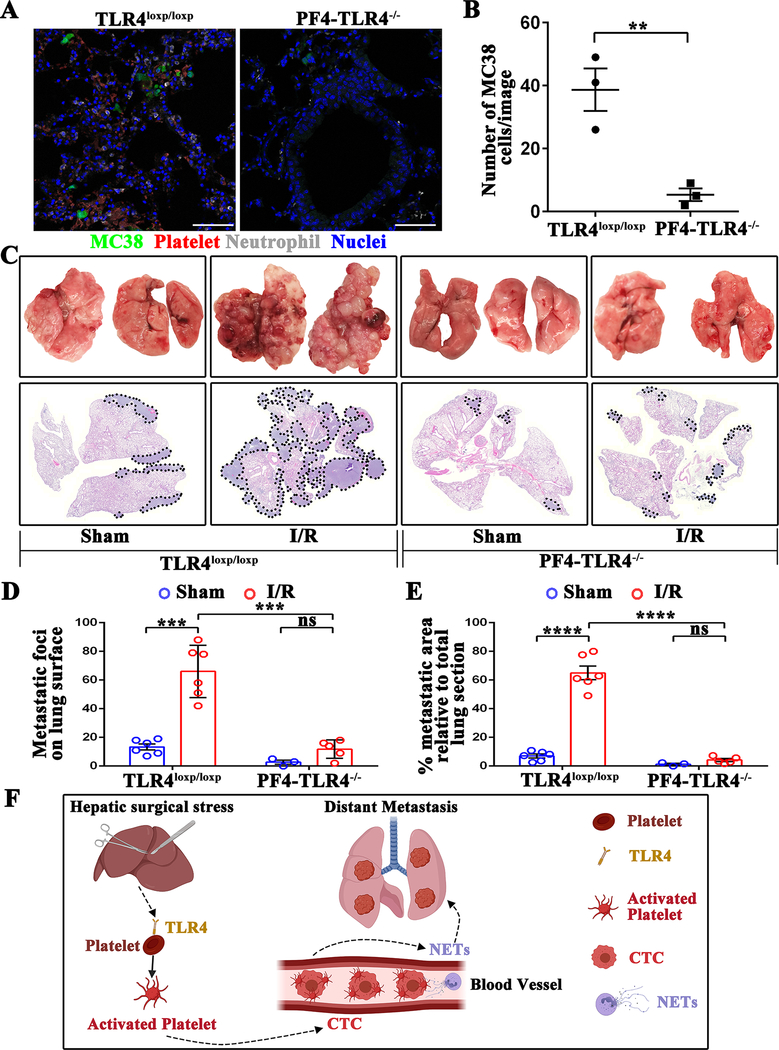
Figure 7.
Platelets-specific TLR4-KO significantly reduces surgical stress-induced metastasis in vivo. (A) Representative immunofluorescence images of confocal microscopy showing tumor cells in the lungs of TLR4loxp/loxp and PF4-TLR4−/− mice at 24 hours following hepatic I/R. Green: MC38; Red: Platelets; Gray: Neutrophil; Blue: Nuclei. Scale bar, 100μm. (B) Quantitation of individual tumor cells in the lungs of TLR4loxp/loxp and PF4-TLR4−/− mice at 24 hours following hepatic I/R. Points represent the mean ± SEM (n ≥ 9 images). (C) Representative images of pulmonary nodules and H&E staining of lung sections after necropsy in TLR4loxp/loxp and PF4-TLR4−/− mice subjected to sham or I/R. (D) Tumor burden as determined by the number of metastatic pulmonary nodules 21 days after tumor cell injection in TLR4loxp/loxp and PF4-TLR4−/− mice subjected to sham or hepatic I/R group. (E) Tumor burden determined in mice from E as a percentage of lung replacement by metastatic tumors. Data are presented as mean ± SEM from n = 3–6 mice per group. ns: not significant, **P<0.01, ***P<0.001 and ****P<0.0001. (F) Schematic diagram of the signaling pathways involved in hepatic I/R induced distant metastasis. Systematic expression of HMGB1 and histone after hepatic I/R activate platelet through TLR4-ERK5-integrin axis, which promotes platelet-tumor cell aggregate formation. Such aggregation facilitates the NET-mediated capture of platelet-tumor cell aggregates and then stimulate tumor cells migration, which promote subsequent tumor distant metastasis.