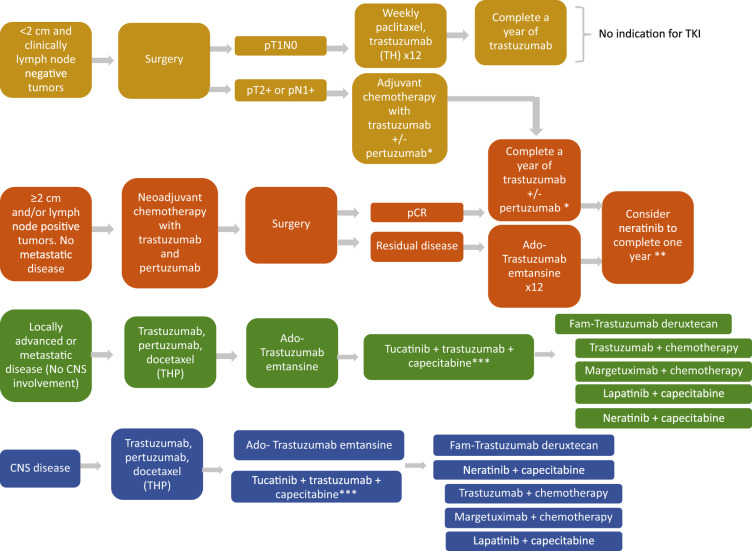
Fig. 3. Proposed treatment algorithm for HER2+Breast Cancer.
This is a suggested treatment algorithm for early stage and advanced human epidermal growth factor 2 positive BC, based on the current United States Food and Drug Administration approvals. Radiation therapy and endocrine therapy should be incorporated when appropriate. There are multiple options for metastatic disease beyond second line, tucatinib can be considered in the third line setting based on the results of the phase 3 HER2CLIMB study. Other agents can be considered as fourth line or beyond, based on prior therapies, toxicity profile and comorbidities. *Subgroup analysis of APHINITY showed that patients with node positive disease have a greater benefit from adjuvant dual HER2 blockade. **Benefit seen in patients with hormone receptor positive and HER2 positive disease. There are no data about the use of neratinib after treatment with pertuzumab and/or ado-trastuzumab emtansine. ***Tucatinib is FDA approved in the second line setting after treatment with trastuzumab, pertuzumab and ado-trastuzumab emtansine. Can be considered for patients with CNS involvement.