FIGURE 2.
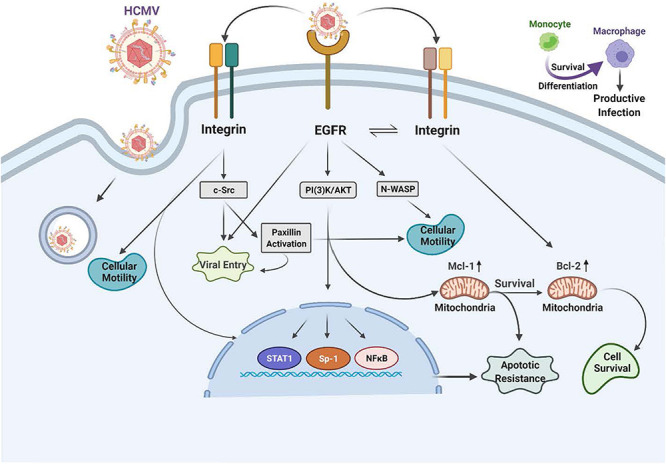
Human cytomegalovirus (HCMV) glycoprotein binding to cognate receptors controls viral signaling, trafficking and the key cellular functions required for productive infection of monocytes. The HCMV glycoproteins bind several cellular receptors, including EGFR and the β1- and β3-integrins. Following binding, these receptor engagements trigger a variety of signal transduction pathways in the infected monocyte. For example, gB binding to EGFR results in activation of the intrinsic EGFR tyrosine kinase and down stream signaling that is required for entry, for the unique multi-vesicular nuclear translocation process seen in monocytes, as well as for motility, and the survival of the monocytes and their differentiation into macrophages. The engagement of the pentameric complex, gH/gL/UL128-131 with β1- and β3-integrins on the surface of monocytes activates c-Src to drive the required signaling pathways needed for entry, nuclear translocation, motility, survival and differentiation. More specifically, this downstream signaling following EGFR and integrin engagement acts in concert to activate numerous down stream signaling pathways, including the NF-κB pathway, PI(3)K, and the MAPK pathway, which culminates in enhanced transactivation of many host cell promoters via increases in transcriptional regulators, altercations in actin regulatory proteins that modulate motility (such as N-WASP and paxillin) and changes in Mcl-1 and Bcl-2 to promote survival of short-lived monocytes.
