Abstract
Calpain I is a calcium-dependent cysteine protease which has dual effects on tissue inflammation depending on its cellular location. Intracellularly, calpain I has pro-inflammatory properties but becomes anti-inflammatory when exteriorised into the extracellular space. In this study, the effect of calpain I on joint pain was investigated using the kaolin/carrageenan model of acute synovitis. Evoked pain behaviour was determined by von Frey hair algesiometry and non-evoked pain was measured using dynamic hindlimb weight bearing. Local administration of calpain I reduced secondary allodynia in the acute inflammation model and this effect was blocked by the cell impermeable calpain inhibitor E-64c. Calpain I also blocked the algesic effect of the protease activated receptor-2 (PAR-2) cleaving enzyme mast cell tryptase. The cell permeable calpain blocker E-64d also produced analgesia in arthritic joints. These data suggest that calpain I produces disparate effects on joint pain viz. analgesia when present extracellularly by disarming PAR-2, and pro-algesic when the enzyme is inside the cell.
Keywords: Arthritis, calpains, inflammation, joint pain, protease activated receptors
Introduction
Rheumatoid arthritis (RA) is a chronic inflammatory joint disease affecting 0.5–1% of the total population.1 RA is characterized by persistent synovitis resulting in swelling, degradation of articular tissues, and pain. Most RA patients are able to manage their disease with Disease Modifying Anti-Rheumatic Drugs (DMARDs) or biologics that block endogenous cytokine activity. Despite these great advances in RA treatment, not all patients show an improvement in disease progression or experience pain relief with mainstream medications. The mechanisms and mediators that promote inflammatory joint pain still require further elucidation.
Proteases are catabolic molecules that breakdown long-chain proteins into shorter polypeptide subunits. In addition to their enzymatic properties, proteases are involved in controlling pain and inflammation.2,3 Calpains are Ca2+-dependent non-lysosomal cysteine proteases that are widely expressed in multiple tissues and contribute to inflammatory processes and pain.4,5 Elevated levels of calpains have been found in the synovial fluid and joint tissues of arthritis patients6–8 and in an animal model of arthritis.9 There are two main isoforms of calpain viz. calpain I (µ-calpain) and calpain II (m-calpain). Calpain I is activated by micromolar concentrations of calcium while calpain II is activated by millimolar concentrations of calcium10; thus, calpain I likely plays a greater role in physiological processes including inflammation and pain.5 Calpains were initially thought to exist and act exclusively within cells to promote inflammation by enhancing, for example, T-lymphocyte migration and inflammatory cytokine secretion.11,12 It has recently been found, however, that calpains can be exteriorised via an ATP-binding cassette transporter (ABCA1) present in lymphocytes.13 Once in the extracellular space, calpains switch their function to promote the activity of anti-inflammatory molecules such as transforming growth factor-beta and chemerin,14,15 as well as inactivating pro-inflammatory cytokines such as interleukin-17.13
The opposing effects of calpain depending on cellular location have been substantiated by the use of inhibitors that can either cross the cell membrane or remain in the extracellular milieu. For example, the cell-permeable irreversible calpain I inhibitor E-64d has been found to suppress IL-6 and IL-17 production in Th cells corroborating a pro-inflammatory effect of calpain within cells.16 Similarly, the calpain inhibitor MDL-28170, which also readily crosses cell membranes, was able to reduce mechanical allodynia in the zymosan model of inflammatory pain.5 Conversely, the membrane non-permeable inhibitor E-64c has been shown to block cell surface calpain activity in worms and prevents the cleavage of extracellular clotting factors.17 In rat striatal muscle, prolonged E-64c treatment inhibited extracellular calpain function and reduced neutrophil accumulation into the damaged tissue.18
A further mechanism by which extracellular calpain can reduce inflammation and pain is by disarming proteinase activated receptor-2 (PAR2).19 The PARs are G protein-coupled receptors that signal following proteolytic cleavage of the extracellular N terminus of the receptor leading to the unveiling of a tethered ligand sequence. The revealed tethered ligand can then dock onto a binding domain located on the second extracellular loop of the same receptor resulting in PAR activation. In knee joints, PAR2 has been localised on approximately 60% of articular afferents where its activation causes nociceptor sensitization and increased leukocyte trafficking.20 Several serine proteinases can activate PAR2 by cleaving the N-terminus at its canonical cleavage site. Intra-articular injection of mast cell tryptase or neutrophil elastase, for example, produce a pro-nociceptive and pro-inflammatory response in joints that is PAR2-dependent.21,22 Other proteinases have been shown to disarm PAR2 by cleaving the receptor at a non-canonical site resulting in receptor inactivation. Cathepsin G, plasmin, and calpain I are among the group of proteinases that are known to disarm PAR2.23
The aim of the present study was to compare the effects of extracellular and intracellular calpain I in a rat model of joint inflammation and to investigate a possible link between calpain I and PAR2.
Methods
Animals
Male Wistar rats (250–350 g; Charles River Laboratories, Senneville, QC, Canada) were housed on ventilated racks at 22 °C ± 2 °C on a 12:12 hours light:dark cycle. After arrival at the animal care facility, the rats were permitted at least one week to acclimate to their environment. Animals were housed in pairs, cages were lined with woodchip bedding, and animals were provided with environmental enrichment. Standard laboratory chow and water were provided ad libitum. All experimental protocols were approved by the Dalhousie University Committee on the Use of Laboratory Animals, which acts in accordance with Animal Research: Reporting of In Vivo Experiments (ARRIVE) and the Canadian Council for Animal Care.
Kaolin and carrageenan synovitis model
Acute synovitis was induced as previously described.24,25 Rats were deeply anesthetized by gaseous anaesthetic (2–4% isoflurane; 100% oxygen at 1 L/min) until abolition of the pedal pinch reflex. The right stifle (knee) joint was shaved, swabbed with 100% ethanol, and knee joint diameter was measured using digital calipers (VWR, Canada) placed laterally across the joint line. Three separate measurements were made, and the diameters averaged. An injection of 1% kaolin (50 µl) was then made into the intra-articular (i.artic.) space of the right knee. The joint was extended and flexed for 10 minutes to cause cartilage debridement. The joint was then injected with 1% carrageenan (50 µl) and the joint was moved for another 30 seconds. At 24 hours, joint diameter was remeasured and pain tests were performed.
Von Frey hair mechanosensitivity
Hindpaw sensitivity to tactile stimulation was blindly assessed using a series of von Frey hair filaments in a modification of the Dixon’s up-down method.26 Animals were placed in elevated Plexiglas chambers on metal mesh flooring and allowed to acclimate until exploratory behaviour ceased. A von Frey hair was applied perpendicular to the plantar surface of the ipsilateral hindpaw with enough force to just slightly bend the hair; the hair was then held in place for three seconds. A positive response (withdrawal, shake or lick of the hind paw) was followed by testing with the next lower strength hair. If there was no response, the next higher strength hair was applied up to a maximum cut-off level (15 g). After the first difference in response was observed, four more measurements were made and the pattern of responses was converted to a 50% withdrawal threshold calculated using the following formula: 10[Xf+kδ]/10,000; where Xf = value (in log units) of the final von Frey hair used, k = tabular value for the pattern of the last six positive/negative responses, and δ = mean difference (in log units) between stimuli. Animals were returned to their home cages for the interval between measurements.
Hindlimb weight bearing
Hindlimb incapacitance was blindly assessed using a dynamic weight bearing (DWB) system (Bioseb, Boulogne, France). Rats were placed in a Perspex chamber (24 cm long x 24 cm wide x 30 cm tall) with a pressure-sensitive floor and a video camera mounted above. A three-minute video was recorded of the animal moving freely around the chamber. Video footage and sensor pad data were then analyzed to determine weightbearing on each of the four limbs. Ipsilateral weightbearing was calculated as a percentage of the total weight on both hindlimbs.
Experimental timelines
Measurements of von Frey hair mechanosensitivity and weight bearing were taken before (naïve) and 24 hours after synovitis induction. The effect of exogenous calpain I on arthritic joint pain was determined by injecting calpain I (1.0 U/50 µl) or vehicle (0.9% saline) subcutaneously over the ipsilateral knee joint 24 hours after arthritis induction. Dose of calpain I was based on a previous report.15 Von Frey hair algesiometry and incapacitance were assessed hourly over the next 5 hours. To verify that the antinociceptive effects of calpain were occurring in the extracellular compartment, a separate group of rats was treated with the cell impermeable calpain I inhibitor E-64c (5 mg/kg i.p.) immediately prior to the administration of calpain I. The dose of E-64c was based on a previous study.27
To investigate the potential role of PAR2 in extracellular calpain-induced responses, experiments were carried out using the canonical PAR2 cleaving proteinase tryptase. Calpain I (1.0 U/50 µl) or vehicle (saline 50 µl) was injected s.c. over the right knee joint of naïve rats 5 minutes before local administration of tryptase (0.15 U/20 µl saline). Dose of tryptase was based on a previous study.21 Pain behaviour was assessed hourly over the next 5 hours.
The effect of intracellular endogenous calpain I on joint pain was tested by administration of the cell permeable calpain I inhibitor E-64d (5 mg/kg i.p.) or vehicle (10% dimethyl sulfoxide/10% cremophor/80% saline i.p.) 30 minutes before (loading dose) and 24 hours (treatment dose) after i.artic. kaolin/carrageenan. The dose of E-64d was based on a previous study.27 Pain behaviour was assessed hourly for the proceeding 5 hours.
At the end of each experiment, all rats were euthanized under isoflurane anaesthesia by cardiac injection of euthansol (0.1 ml, 340 mg/ml sodium pentobarbital).
Drugs and reagents
Calpain I (isolated form human erythrocytes), kaolin, carrageenan, cremophor, dimethyl sulphoxide (DMSO), tryptase (from human lung) and urethane were obtained from Sigma-Aldrich (St. Louis, MO, USA). E-64c (2S,3S)-3-[[(2S)-4-methyl-1–(3-methylbutylamino)-1-oxopentan-2-yl]carbamoyl]oxirane-2-carboxylic acid) was purchased from ApexBio (Huston, Texas, USA) and E-64d (2S,3S)-trans-Epoxysuccinyl-L-leucylamido-3-methylbutane ethyl ester) was purchased from AdooQ Bioscience (Irvine, CA, USA). E-64c and E-64d were prepared in vehicle (1:1:8; DMSO: cremophor: saline) on the day of use. Isoflurane and euthansol were purchased from CDMV (Dartmouth, NS, Canada).
Statistical analysis
All data are expressed as mean ± SEM. Two-group comparisons were analyzed with a paired Student t-test for before and after treatment comparisons. The tryptase time course was analysed by one-way repeated measures ANOVA with a Dunnett’s post hoc multiple comparisons test. Time course data were analyzed using two-way repeated measures ANOVA with a Holm-Sidak post hoc test when comparing different groups. A P value less than 0.05 was considered statistically significant.
Results
Effect of calpain I on joint nociception
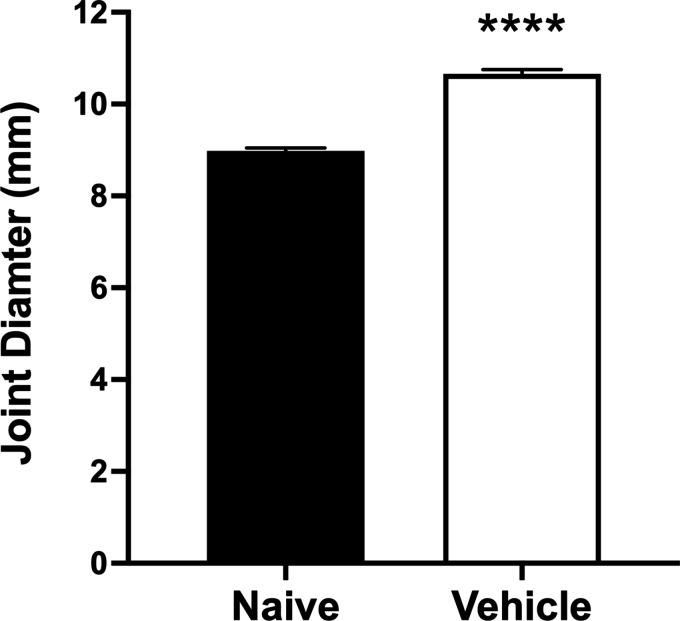
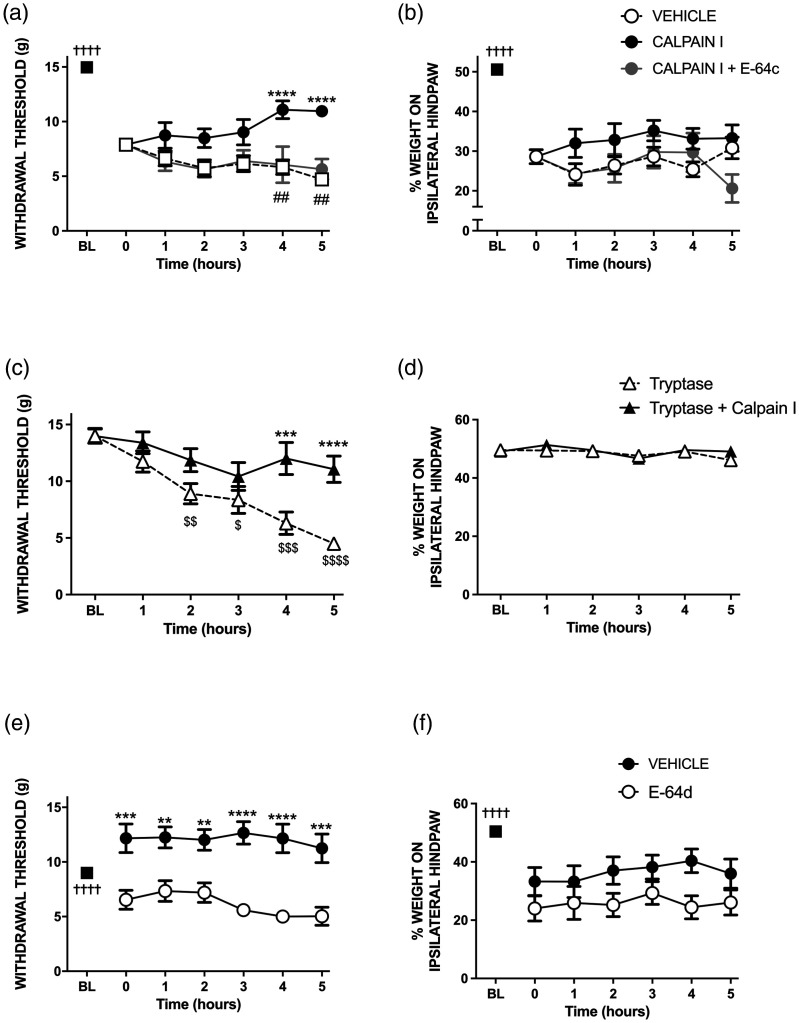
Twenty-four hours after kaolin/carrageenan injection, rat knee joints were inflamed as evidenced by an increase in joint diameter of 18% (P < 0.0001, two-tailed paired Student t-test, n = 30, Figure 1). Arthritic animals showed signs of pain (Figure 2(a) and (b)) since paw withdrawal threshold decreased by 47% (P < 0.0001, n = 30), and ipsilateral weight bearing fell by 43% (P < 0.0001, n = 30) compared to baseline. Local (s.c.) administration of calpain I attenuated this secondary allodynia (P < 0.0001, two-way ANOVA, n = 10; Figure 2(a)), but had no effect on hindlimb incapacitance (P = 0.21, Figure 2(b)) over the 5 hr time-course when compared to an injection of vehicle (n = 14).
Figure 1.
Increase in knee joint diameter following intra-articular injection of 1% kaolin/carrageenan confirming joint inflammation. ****P < 0.0001 two-tailed paired Student t-test. Data are mean values ± SEM.
Figure 2.
The effect of local injection of calpain I on hindpaw withdrawal threshold (a) and hindlimb weight bearing (b) in acutely inflamed rat knees. The antiallodynic effect of calpain I was blocked by the cell impermeable inhibitor E-64c. Treatment of naïve knees with mast cell tryptase produced secondary allodynia (c), but had no effect on hindlimb weight-bearing (d). The protease-induced pain response was attenuated 4–5 hours after treatment with calpain I. In acute synovitis animals, the cell permeable calpain inhibitor E-64d produced a secondary anti-allodynic response (e); E-64d had no effect on hindlimb incapacitance (f). †††† P < 0.0001 two-tailed paired Student t-test between baseline (BL) and t = 0 hr. **P < 0.01, ***P < 0.001, ****P < 0.0001 two-way repeated measures ANOVA with Holm-Sidak multiple comparison test of treatment versus vehicle. $P < 0.01, $$P < 0.01, $$$P < 0.001, P < 0.0001 one-way repeated measures ANOVA with Dunnett’s multiple comparison test. ##P < 0.001 two-way repeated measures ANOVA with Holm-Sidak multiple comparisons test of calpain I versus calpain I + E-64c. Data are mean values ± SEM.
Treatment of the inflamed knee with the cell impermeable calpain I blocker E-64c (s.c.) significantly inhibited the anti-nociceptive effect of extracellular calpain I when assessed by von Frey hair algesiometry (P < 0.0001, n = 6, Figure 2(a)). In contrast, E-64c had no effect on calpain I responses with respect to hindlimb weight bearing (Figure 2(b)).
Inhibition of tryptase-induced pain by calpain I
Subcutaneous administration of the serine protease tryptase over the knees (s.c.) of naïve rats caused a gradual reduction in hindpaw withdrawal threshold over a five-hour period (P < 0.0001, one way ANOVA, Figure 2(c)). Pre-treatment with calpain I attenuated the secondary allodynic effect of tryptase over the time course (P < 0.01, n = 9). Tryptase had no effect on hindlimb weight bearing in these experiments (Figure 2(d)).
Intracellular calpain I blockade effects on joint nociception
Prophylactic treatment (s.c. over the knee) of arthritic animals with the cell-permeable inhibitor of intracellular calpain I, E-64d, significantly reduced kaolin/carrageenan-induced tactile secondary allodynia across the 5 hour time-course (P < 0.0001, Figure 2(e)) while hindlimb weight bearing was unaffected by E-64d (P = 0.08, Figure 2(f)) when compared to vehicle.
Discussion
Elevated calpain I levels in arthritic joints are known to contribute to the break-down of articular cartilage by hydrolysing aggrecans and proteoglycans. In other organs, calpain I has been shown to have opposing effects on tissue inflammation depending upon whether the enzyme is present in the intracellular or extracellular compartment. In the present investigation, it was found that extracellular calpain was analgesic in acutely inflamed rat knee joints, while intracellular calpain appeared to have pro-algesic properties. These findings highlight the complex function of calpain I in pain and inflammation that is dependent upon the cellular location of the enzyme.
The role of calpain in mediating pain has primarily focussed on neuropathic pain as it causes proteolysis of myelin basic protein leading to neuronal damage.28 The role of calpain in inflammatory pain, however, is unclear. Carrageenan-induced inflammation of the hindpaw has been shown to cause more than a doubling in calpain activity in both peripheral and central neurones on the ipsilateral side.29 Here, kaolin/carrageenan produced a pain response 24-hours after intra-articular injection as evidenced by a reduction in hindpaw withdrawal threshold and a shift in hindlimb weight bearing. Tactile secondary allodynia was blocked by treating the animals with calpain I. The action of calpain was itself inhibited by the cell impermeable inhibitor E-64c confirming that the analgesic effect of calpain I was occurring in the extracellular space.
One of the possible mechanisms by which extracellular calpain I produced analgesia in the synovitis model could be by disarming PAR2 which is known to be pro-algesic in joints.20 Articular administration of PAR2 cleaving tryptase caused secondary allodynia as previously reported21 and this pain response was attenuated by pre-treatment with calpain I. It is known that calpain I can hydrolyse the N terminus of PAR2 at a locus upstream of the canonical cleavage site.23 Thus, one explanation for calpain I inhibiting tryptase-induced allodynia could be the disarmament of PAR2 by removal of the N-terminus containing the tethered ligand.
Intracellular blockade of calpain I activity with the cell permeable inhibitor E-64d resulted in an analgesic response in the knee synovitis model. This finding provides indirect evidence that intracellular calpain is pro-algesic. Pain assessments in the zymosan model of acute inflammation also found that another cell permeable calpain inhibitor (MDL-28170) was analgesic when administered peripherally into the hindpaw.5 In contrast, Xie et al.30 reported that E-64d had no effect on chronic inflammatory pain indicating that intracellular calpain may only be involved in the development of acute inflammatory pain.
The nociceptive responses observed in this study were confined to von Frey hair algesiometry and had no influence on weight bearing deficits. This may be because these two techniques interrogate different types of pain i.e. reflexive versus non-reflexive behaviours respectively. It appears that the articular calpain system may only be effective at modulating secondary allodynia and is not involved in normalising gait.
In conclusion, the present study discovered that in acutely inflamed knee joints, extracellular calpain I can reduce joint pain, possibly by deactivating articular PAR2. In contrast, the biological function of intracellular calpain I is to enhance joint pain. Thus, calpain inhibitors that are cell permeable could provide a novel target for alleviating acute synovitis pain.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by a project grant from the Canadian Institutes for Health Research.
ORCID iD: Jason J McDougall https://orcid.org/0000-0003-4529-3428
References
- 1.Scott DL, Wolfe F, Huizinga TW. Rheumatoid arthritis. Lancet 2010; 376: 1094–1108. [DOI] [PubMed] [Google Scholar]
- 2.McDougall JJ, Muley MM. The role of proteases in pain. Handb Exp Pharmacol 2015; 227: 239–260. [DOI] [PubMed] [Google Scholar]
- 3.Steinhoff M, Buddenkotte J, Shpacovitch V, Rattenholl A, Moormann C, Vergnolle N, Luger TA, Hollenberg MD. Proteinase-activated receptors: transducers of proteinase-mediated signaling in inflammation and immune response. Endocr Rev 2005; 26: 1–43. [DOI] [PubMed] [Google Scholar]
- 4.Goll DE, Thompson VF, Li H, Wei W, Cong J. The calpain system. Physiol Rev 2003; 83: 731–801. [DOI] [PubMed] [Google Scholar]
- 5.Kunz S, Niederberger E, Ehnert C, Coste O, Pfenninger A, Kruip J, Wendrich TM, Schmidtko A, Tegeder I, Geisslinger G. The calpain inhibitor mdl 28170 prevents inflammation-induced neurofilament light chain breakdown in the spinal cord and reduces thermal hyperalgesia. Pain 2004; 110: 409–418. [DOI] [PubMed] [Google Scholar]
- 6.Yamamoto S, Shimizu K, Shimizu K, Suzuki K, Nakagawa Y, Yamamuro T. Calcium-dependent cysteine proteinase (calpain) in human arthritic synovial joints. Arthritis Rheum 1992; 35: 1309–1317. [DOI] [PubMed] [Google Scholar]
- 7.Fukui I, Tanaka K, Murachi T. Extracellular appearance of calpain and calpastatin in the synovial fluid of the knee joint. Biochem Biophys Res Commun 1989; 162: 559–566. [DOI] [PubMed] [Google Scholar]
- 8.Suzuki K, Shimizu K, Hamamoto T, Nakagawa Y, Hamakubo T, Yamamuro T. Biochemical demonstration of calpains and calpastatin in osteoarthritic synovial fluid. Arthritis Rheum 1990; 33: 728–732. [DOI] [PubMed] [Google Scholar]
- 9.Szomor Z, Shimizu K, Fujimori Y, Yamamoto S, Yamamuro T. Appearance of calpain correlates with arthritis and cartilage destruction in collagen induced arthritic knee joints of mice. Ann Rheum Dis 1995; 54: 477–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cong J, Goll DE, Peterson AM, Kapprell HP. The role of autolysis in activity of the Ca2+-dependent proteinases (mu-calpain and m-calpain). J Biol Chem 1989; 264: 10096–10103. [PubMed] [Google Scholar]
- 11.Butler JT, Samantaray S, Beeson CC, Ray SK, Banik NL. Involvement of calpain in the process of jurkat T cell chemotaxis. J Neurosci Res 2009; 87: 626–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Imam SA, Guyton MK, Haque A, Vandenbark A, Tyor WR, Ray SK, Banik NL. Increased calpain correlates with Th1 cytokine profile in PBMCs from MS patients. J Neuroimmunol 2007; 190: 139–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Perez J, Dansou B, Hervé R, Levi C, Tamouza H, Vandermeersch S, Demey-Thomas E, Haymann J-P, Zafrani L, Klatzmann D, Boissier M-C, Letavernier E, Baud L. Calpains released by T lymphocytes cleave TLR2 to control IL-17 expression. J Immunol 2016; 196: 168–181. [DOI] [PubMed] [Google Scholar]
- 14.Cash JL, Hart R, Russ A, Dixon JPC, Colledge WH, Doran J, Hendrick AG, Carlton MBL, Greaves DR. Synthetic chemerin-derived peptides suppress inflammation through ChemR23. J Exp Med 2008; 205: 767–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abe M, Oda N, Sato Y. Cell-associated activation of latent transforming growth factor-beta by calpain. J Cell Physiol 1998; 174: 186–193. [DOI] [PubMed] [Google Scholar]
- 16.Iguchi-Hashimoto M, Usui T, Yoshifuji H, Shimizu M, Kobayashi S, Ito Y, Murakami K, Shiomi A, Yukawa N, Kawabata D, Nojima T, Ohmura K, Fujii T, Mimori T. Overexpression of a minimal domain of calpastatin suppresses IL-6 production and Th17 development via reduced NF-kappaB and increased STAT5 signals. PLoS One 2011; 6: e27020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang Q, Da'dara AA, Skelly PJ. The human blood parasite Schistosoma mansoni expresses extracellular tegumental calpains that cleave the blood clotting protein fibronectin. Sci Rep 2017; 7: 12912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Raj DA, Booker TS, Belcastro AN. Striated muscle calcium-stimulated cysteine protease (calpain-like) activity promotes myeloperoxidase activity with exercise. Pflugers Arch 1998; 435: 804–809. [DOI] [PubMed] [Google Scholar]
- 19.Loew D, Perrault C, Morales M, Moog S, Ravanat C, Schuhler S, Arcone R, Pietropaolo C, Cazenave JP, van Dorsselaer A, Lanza F. Proteolysis of the exodomain of recombinant protease-activated receptors: prediction of receptor activation or inactivation by MALDI mass spectrometry. Biochemistry 2000; 39: 10812–10822. [DOI] [PubMed] [Google Scholar]
- 20.Russell FA, Schuelert N, Veldhoen VE, et al. Proteinase-activated receptor-2 (PAR(2)) activation sensitises primary afferents and causes leukocyte rolling and adherence in the rat knee joint. Br J Pharmacol 2012; 167: 1665–1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Borbély É, Sándor K, Markovics A, Kemény Á, Pintér E, Szolcsányi J, Quinn JP, McDougall JJ, Helyes Z. Role of capsaicin-sensitive nerves and tachykinins in mast cell tryptase-induced inflammation of murine knees. Inflamm Res 2016; 65: 725–736. [DOI] [PubMed] [Google Scholar]
- 22.Muley MM, Reid AR, Botz B, Bölcskei K, Helyes Z, McDougall JJ. Neutrophil elastase induces inflammation and pain in mouse knee joints via activation of proteinase-activated receptor-2. Br J Pharmacol 2016; 173: 766–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Adams MN, Ramachandran R, Yau M-K, Suen JY, Fairlie DP, Hollenberg MD, Hooper JD. Structure, function and pathophysiology of protease activated receptors. Pharmacol Ther 2011; 130: 248–282. [DOI] [PubMed] [Google Scholar]
- 24.Heppelmann B, Pawlak M. Sensitisation of articular afferents in normal and inflamed knee joints by substance P in the rat. Neurosci Lett 1997; 223: 97–100. [DOI] [PubMed] [Google Scholar]
- 25.McDougall JJ, Yu V, Thomson J. In vivo effects of CB2 receptor-selective cannabinoids on the vasculature of normal and arthritic rat knee joints. Br J Pharmacol 2008; 153: 358–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods 1994; 53: 55–63. [DOI] [PubMed] [Google Scholar]
- 27.Yoshifuji H, Umehara H, Maruyama H, Itoh M, Tanaka M, Kawabata D, Fujii T, Mimori T. Amelioration of experimental arthritis by a calpain-inhibitory compound: regulation of cytokine production by E-64-d in vivo and in vitro. Int Immunol 2005; 17: 1327–1336. [DOI] [PubMed] [Google Scholar]
- 28.Yuan X-C, Wu C-H, Gao F, Li H-P, Xiang H-C, Zhu H, Pan X-L, Lin L-X, Liu Y-S, Yu W, Tian B, Meng X-F, Li M. Activation and expression of mu-calpain in dorsal root contributes to RTX-induced mechanical allodynia. Mol Pain 2017; 13: 1744806917719169–1744806917719107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pareek TK, Keller J, Kesavapany S, Pant HC, Iadarola MJ, Brady RO, Kulkarni AB. Cyclin-dependent kinase 5 activity regulates pain signaling. Proc Natl Acad Sci U S A 2006; 103: 791–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xie W, Uchida H, Nagai J, Ueda M, Chun J, Ueda H. Calpain-mediated down-regulation of myelin-associated glycoprotein in lysophosphatidic acid-induced neuropathic pain. J Neurochem 2010; 113: 1002–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]