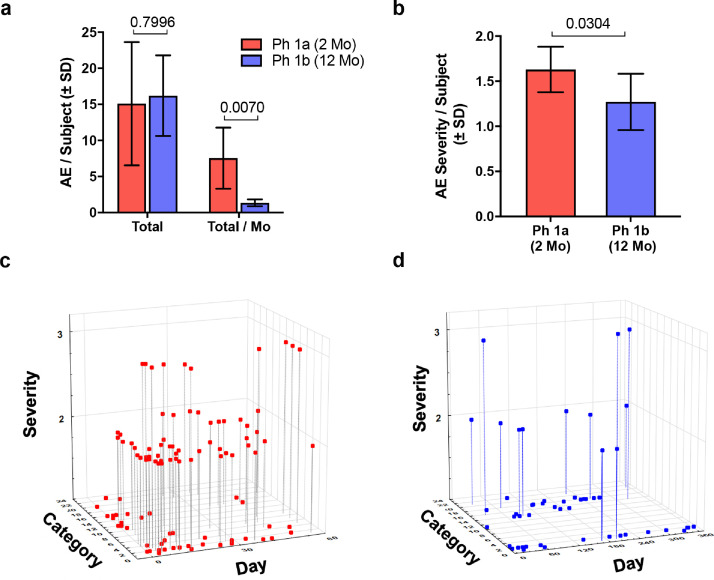
Fig. 1.
Adverse events (AE) comparing two clinical trials of sargramostim. PD subjects were administered sargramostim (Leukine®, human recombinant GM-CSF) in a previous Phase 1a (Ph 1a) (n = 10) and current Phase 1b (Ph 1b) clinical trial (n = 5). Subjects in the Ph 1a trial received 6 μg/kg of sargramostim every day for 2 months. In a proof-of-concept study to attenuate AE frequency and severity and extend administration, subjects in the Ph 1b trial received 3 μg/kg sargramostim on a 5 day on/2 day off regimen for 12 months. (a) Total number of adverse events (AEs) per subject recorded during the 2- and 12- month interventional period (Total) normalized on a monthly basis (Total/Mo). (b) AE severity scored on a scale of 1–3 severity as (1) mild, (2) moderate, or (3) severe. Mild events cause minimal discomfort or concern, may require minimal or no treatment, and do not interfere with daily activities. Moderate events were defined as causing discomfort, inconvenience, or concerns which were ameliorated with simple therapeutic measures. Severe adverse events were defined as causing discomfort or incapacitation that require prescription drug therapy or other treatments or interventions by medical personnel. Differences in means (± SD) for Total AE/Subject, Total AE/Subject/Mo, and Severity of AEs between Ph Ia vs Ph 1b trials were determined by Student's t-test and p values annotated above the pair-wise comparisons. (c) Graphical representation displaying reported AE based on AE severity (y axis), AE category (z axis), and day of treatment (x axis) for the Ph 1a trial. (d) Graphical representation displaying reported AE based on AE severity (y axis), AE category (z axis), and day of treatment (x axis) for the Ph 1b study. (c and d) AE categories are defined in Table 2.