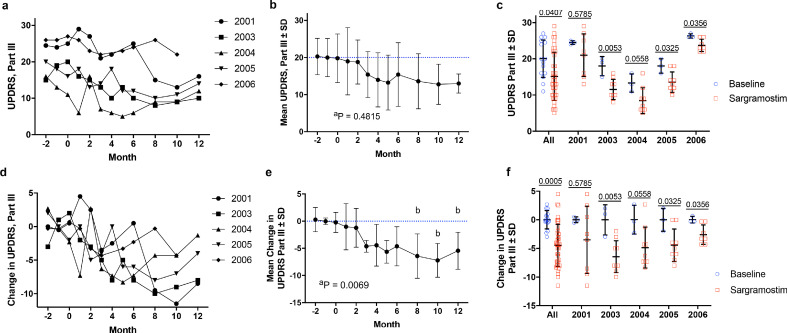
Fig. 2.
MDS-Unified Parkinson's Disease Rating Scale (UPDRS) Part III motor assessment before and during sargramostim treatment. Prior to treatment, subjects (n = 5) underwent at least three separate baseline evaluations (at month -4, -3, -2, and/or -1 before initiation) and then began drug administration (3 ug/kg per day, 5 days on/2 days off). After treatment initiation, subjects were evaluated by the study neurologist once/month for 6 months and once/ every 2 months thereafter for 12 months. (a) Raw UPDRS Part III scores over time grouped for individual subjects (2001, 2003, 2004, 2005, 2006). (b) Total mean UPDRS Part III scores grouped by time of treatment. Blue dashed lines indicate mean baseline measurement. (c) Mean UPDRS Part III scores ± SD grouped by combined (All) and individual subjects. Specific p values are indicated above each subject. Baseline values are represented as blue circles and sargramostim treatment is represented as red squares. (d) Change from baseline in UPDRS Part III scores over time grouped for individual subjects. (e) Mean change from baseline ± SD in UPDRS Part III scores grouped by time of treatment. (f) Mean change from baseline in UPDRS Part III scores ± SD grouped by combined (All) and individual subjects. Specific p values are indicated above each subject. Baseline values are represented as blue circles and sargramostim treatment is represented as red squares. Significant differences (± SD) in baseline and treated means were determined by Student's t-test with p values denoted above comparisons. Differences in means ± SD over time were also determined by one-way ANOVA where p ≤ 0.05 compared to baseline (b).