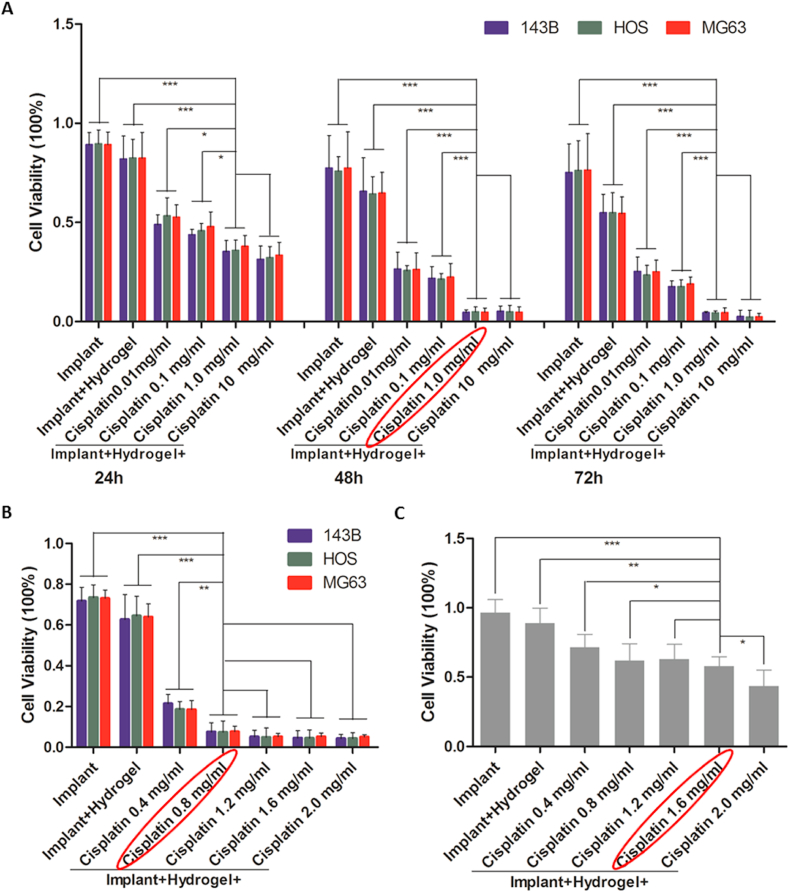
Fig. 2.
In vitro anti-tumour effect and biosafety of the cisplatin/hydrogel-loaded 3D-printed Ti6Al4V implants. (A) The viability of osteosarcoma cells (143B, HOS, and MG63) after incubation with bare implants, hydrogel-loaded implants, and 0.01, 0.1, 1.0, 10 mg/mL cisplatin/hydrogel-loaded implants for 24, 48, and 72 h. (B) The viability of osteosarcoma cells (143B, HOS, and MG63) after incubation with bare implants, hydrogel-loaded implants, and 0.4, 0.8, 1.2, 1.6, 2.0 mg/mL cisplatin/hydrogel-loaded implants for 48 h. (C) The viability of primary human osteoblasts after incubation with bare implants, hydrogel-loaded implants, and 0.4, 0.8, 1.2, 1.6, 2.0 mg/mL cisplatin/hydrogel-loaded implants for 48 h. Data are represented as mean ± standard deviation (n = 6). *p < 0.05, **p < 0.01, ***p < 0.001.