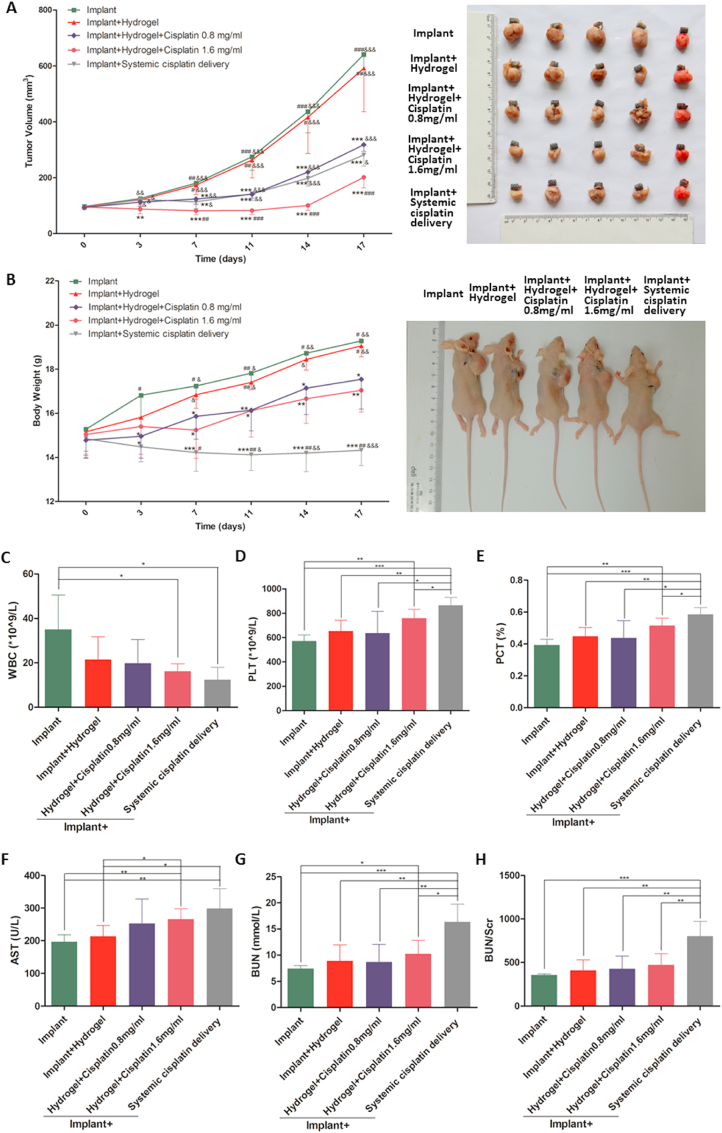
Fig. 3.
In vivo anti-tumour effect and biosafety of the cisplatin/hydrogel-loaded 3D-printed Ti6Al4V implants. Human osteosarcoma 143B cells were inoculated subcutaneously into BALB/c nude mice; tumour progression was monitored. The tumour volume (A) and body weight of mice (B) were measured twice a week throughout the experiment. The blood routine and biochemical parameters (C–H) were determined immediately after sacrificing the mice. Systemic cisplatin delivery via tail intravenous injection of 3 μg of cisplatin per gram of mice body weight twice a week. Data are represented as mean ± standard deviation (n = 5). (A–B) *p < 0.05, **p < 0.01, ***p < 0.001 compared with the implant group; #p < 0.05, ##p < 0.01, ###p < 0.001 compared with the implant + hydrogel + cisplatin 0.8 mg/mL group; &p < 0.05, &&p < 0.01, &&&p < 0.001 compared with the implant + hydrogel + cisplatin 1.6 mg/mL group. (C–H) *p < 0.05, **p < 0.01, ***p < 0.001.