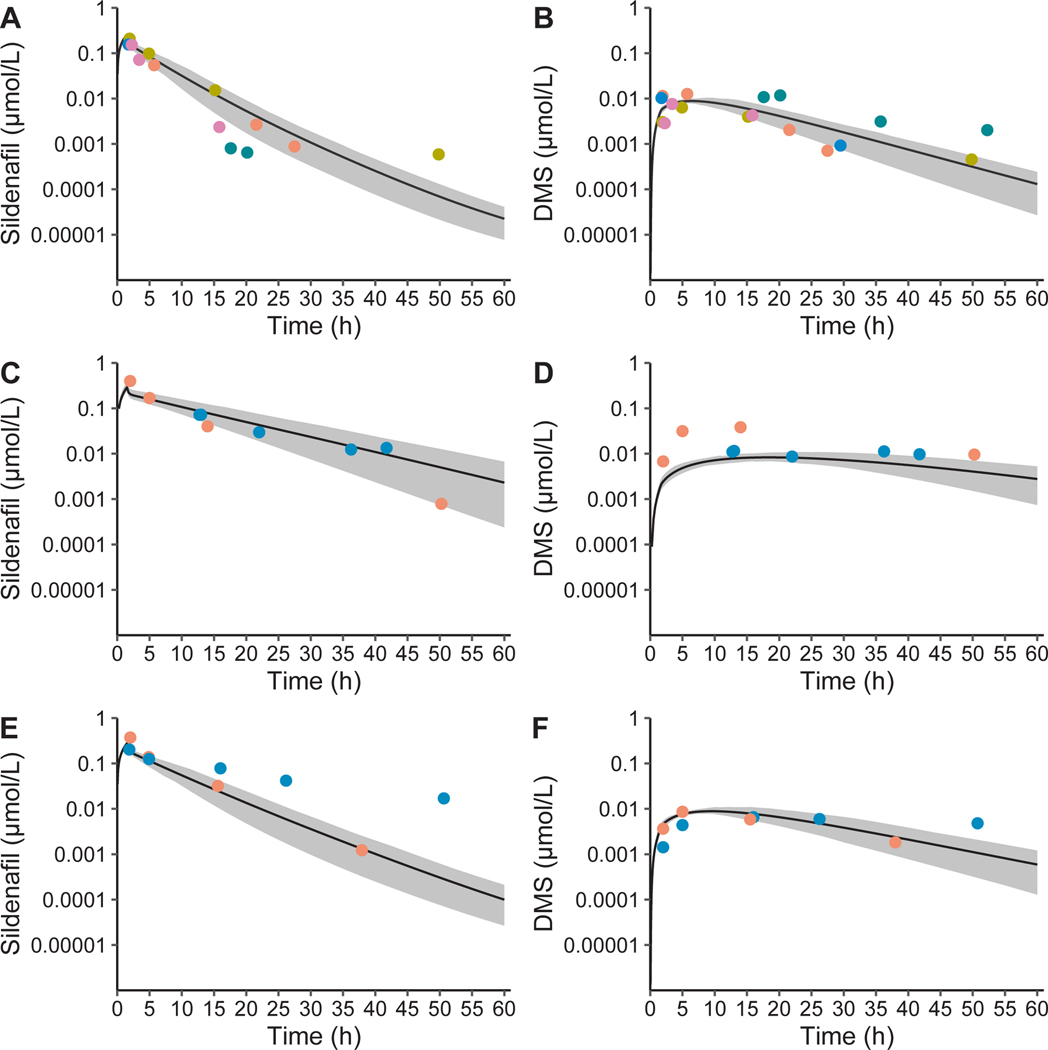
Figure 1.
Sildenafil and N-desmethyl sildenafil (DMS) with and without fluconazole physiologically-based pharmacokinetic model population simulations in preterm infants. Population simulations in 100 preterm infants (33% girls, 7–40 days postnatal age, 24–27 weeks gestational age, and 590–1,242 g) for sildenafil (a) and DMS (b) in infants receiving sildenafil alone, and for sildenafil (c) and DMS (d) in infants receiving sildenafil with steady-state administration of fluconazole for treatment (12 mg/kg i.v. daily) and for sildenafil (e) and DMS (f) in infants receiving sildenafil with fluconazole for prophylaxis (6 mg/kg i.v. every 72 hours). A single dose of 0.25 mg/kg i.v. sildenafil with 6 mg/kg fluconazole i.v. in preterm infants resulted in a simulated mean fold-change of 1.08 for maximal concentration (Cmax) and 1.40 for the area under the curve extrapolated to infinity (AUC0–∞) for sildenafil plus DMS accounting for different phosphodiestesterase type 5 inhibitory activity and protein binding (sildenafil + 0.5*1.25*DMS). A single dose of 0.25 mg/kg i.v. sildenafil with 6 days of fluconazole dosing of 12 mg/kg fluconazole i.v. in preterm infants resulted in a simulated mean fold-change of 1.13 for Cmax and 2.59 for AUC0–∞ for sildenafil plus DMS. The solid grey area is the 95% prediction interval and the dots are concentrations colored by individuals. Results were obtained using the default PK-Sim® ontogeny functions for alpha-1-acid glycoprotein without additional variability introduced on the fraction unbound. Observed concentrations were dose normalized to 0.25 mg/kg.