Abstract
Background.
Nonuse (NU) after stroke is characterized by failure to use the contralesional arm despite adequate capacity. It has been suggested that NU is a consequence of the greater effort and/or attention required to use the affected limb, but such accounts have not been directly tested, and we have poor understanding of the predictors of NU.
Objective.
We aimed to provide preliminary evidence regarding demographic, neuropsychological (ie, apraxia, attention/arousal, neglect), and psychological (ie, self-efficacy) factors that may influence NU in chronic stroke.
Methods.
Twenty chronic stroke survivors with mild to moderate sensory-motor impairment characterized by the Upper-Extremity Fugl-Meyer (UEFM) were assessed for NU with a modified version of the Actual Amount of Use Test (AAUT), which measures the disparity between amount of use in spontaneous versus forced conditions. Participants were also assessed with measures of limb apraxia, spatial neglect, attention/arousal, and self-efficacy. Using stepwise multiple regression, we determined which variables predicted AAUT NU scores.
Results.
Scores on the UEFM as well as attention/arousal predicted the degree of NU (P < .05). Attention/arousal predicted NU above and beyond UEFM (P < .05).
Conclusions.
The results are consistent with the importance of attention and engagement necessary to fully incorporate the paretic limb into daily activities. Larger-scale studies that include additional behavioral (eg, sensation, proprioception, spasticity, pain, mental health, motivation) and neuroanatomical measures (eg, lesion volume and white matter connectivity) will be important for future investigations.
Keywords: stroke, upper-extremity paresis, arousal, arm nonuse, attention, self-efficacy, apraxia, neglect
Introduction
One of the most vexing problems in neurorehabilitation is the phenomenon of arm nonuse (NU). After a stroke, the contralesional arm may exhibit functional motor and sensory impairments that include muscle weakness and reduced proprioception and tactile sensation. These deficits lead to immobility, which is in turn related to spasticity, stiffness, pain, and abnormal reflex mechanisms.1 As a further consequence, many patients exhibit reduced use of the contralesional arm, whether via a maladaptive learning process in which movement is suppressed over time2 or via a more direct stroke-related reduction in volitional drive toward limb use.3
Irrespective of its precise underlying mechanism from a physiological perspective, arm NU is likely to negatively influence clinical and rehabilitation research efforts. It is frequently assumed that measured improvements in capacity as a result of practice will translate to increased use of the arm in daily activities.4 However, recent evidence suggests that despite gains in capacity as a result of spontaneous recovery or rehabilitation training, there may be little to no improvement in use of the arm in daily life. For example, a secondary analysis from a phase II dose-response trial conducted at Washington University aimed to assess changes in the performance of 78 participants with chronic stroke during daily activities over an 8-week intervention period. The investigators used accelerometers to assess overall arm use, movement intensity, or acceleration parameters at weekly intervals. Neither changes in capacity, as measured by the Action Research Arm Test, nor overall amount of practice influenced the amount of daily arm use as measured by accelerometer variables.4 Baseline arm capacity, stroke chronicity, concordance (dominant side = affected side), and activities of daily living status all influenced the intercepts but not the slopes of these accelerometer variables. These data reinforce the importance of assessing capacity and performance separately.5–7 Furthermore, Stewart and Cramer8 highlight the importance of using patient-reported measures for providing insights into motor function after stroke. This observation influenced the choice of measures used here.
NU was the impetus for the development of constraint-induced movement therapy (CIMT), an intervention that combines restraint of the less-affected upper extremity with forced training of the affected extremity using a behavioral shaping protocol. CIMT shows evidence of efficacy in improving arm function as compared with traditional rehabilitation in chronic stroke9–14; however, its short-term efficacy in the acute and subacute phases of stroke is variable.15
Initial development of CIMT was based on a model of conditioning, informed by the observation that monkeys prevented from experiencing early failures did not develop NU.16 On this model, CIMT achieved via an extrinsic factor—a constraint or mitt—extinguishes learned NU. The extrinsic model makes no clear predictions about individual characteristics that would predict NU phenomena. Alternative models, however, suggest that NU is a consequence of intrinsic factors, including the greater effort (eg, force or distance commands) and/or attention (eg, to proprioceptive, somatosensory, and visual feedback) required to use the affected limb. Spontaneous use may, therefore, reflect a trade-off between effort/attention and disability, wherein the unaffected limb will be used alone whenever this may lead to a reasonably successful outcome.17 On this account, and based on models that emphasize intrinsic motivational factors (eg, OPTIMAL18), overcoming NU requires that we address decreased attention, effort, and/or motivation.*
In this context, it is relevant to note that CIMT with a transfer package that may improve attention, effort, and motivation, including a behavioral agreement, diary use, problem-solving to overcome perceived barriers, home skill practice assignments, and weekly telephone calls, has shown superior efficacy to CIMT alone for performance-based behavioral13,19 and structural brain changes.20
Other individual characteristics that may plausibly influence NU include the hemisphere of stroke and, related to this, the common neuropsychological syndromes associated with right- versus left-hemisphere lesions. The spatial neglect syndrome, observed frequently after right-hemisphere stroke, is characterized by both lateralized and nonlateralized attention/arousal deficits and is a strong predictor of stroke outcome (eg, Katz et al21). The nonlateralized attention deficits observed in the neglect syndrome may be particularly relevant to reduced effort and motivation, which are similarly more common in right- than left-hemisphere stroke (see Van Dalen et al22 for review). A disorder that is disproportionately observed in left-hemisphere stroke, limb apraxia, may also contribute to NU. Limb apraxia is characterized by spatio-temporal deficits in imitation, pantomime of tool use movements, and/or tool use, even with the ipsilesional limb, and is a potent predictor of disability after left-hemisphere stroke (eg, Sundet et al23).
Finally, another intrinsic factor that may influence NU is confidence in one’s ability to use the impaired limb (also known as self-efficacy). Self-efficacy has been shown to be modestly correlated with motor capacity24 and to improve in response to treatment (eg, Sugg et al25). Recently, self-efficacy has been shown to predict speed and accuracy of aiming movements to targets,26 yet it is not known whether reduced self-efficacy predicts reduced willingness to use a limb that has adequate capacity.
An important step in the development of targeted treatments for failure to use the limb despite adequate functional ability is a better understanding of the characteristics of individuals who exhibit this phenomenon. In this preliminary study, we carefully selected a representative sample of individuals with chronic stroke who exhibited a range of sensory-motor impairments to provide preliminary evidence regarding some of the demographic, neuropsychological (ie, apraxia, attention/arousal, neglect), and psychological (ie, self-efficacy) factors that may influence NU behavior.
Methods
Participants
Participants were 20 individuals with chronic stroke (>12 months poststroke), 10 of whom had suffered a single left-hemisphere stroke (LCVA) and 10 of whom had experienced a single right-hemisphere stroke (RCVA). Table 1 provides demographic information. Five LCVA and 5 RCVA patients were recruited from the Neurocognitive Rehabilitation Research Registry at Moss Rehabilitation Research Institute (MRRI)27; the remaining 5 LCVA and 5 RCVA patients were recruited from the Registry for Aging and Rehabilitation Evaluation database of the Motor Behavior and Neurorehabilitation Laboratory at the University of Southern California (USC). All were right-handed and achieved a score on the Upper-Extremity Fugl-Meyer (UEFM) of at least 29, demonstrating moderate to mild motor impairment.28,29 Patients with a history of psychosis, neurological disorder, traumatic brain injury, or alcohol/drug abuse were excluded. Participants at the 2 sites were matched for age [t(18) = 0.06; P = .95], chronicity [t(18) = −0.41; P = .68], and UEFM scores [t(18) = .56; P = .58]; the USC sample tended to be slightly more highly educated than the MRRI sample [t(18) = −1.99; P = .06].
Table 1.
Demographic Information.a
| Participant | Site | Stroke Hemisphere |
Age (years) | Education (years) | Years Poststroke | Fugl-Meyer Score |
|---|---|---|---|---|---|---|
| SI | USC | Left | 56 | 16 | 7.08 | 30 |
| S2 | USC | Left | 48 | 12 | 4.76 | 37 |
| S3 | USC | Left | 58 | 14 | 3.08 | 52 |
| S4 | USC | Right | 63 | 14 | 3.27 | 36 |
| S5 | USC | Right | 73 | 16 | 12.04 | 39 |
| S6 | USC | Left | 50 | 18 | 5.52 | 51 |
| S7 | USC | Right | 72 | 16 | 6.62 | 47 |
| S8 | USC | Left | 71 | 12 | 2.19 | 55 |
| S9 | USC | Right | 66 | 13 | 9.29 | 51 |
| S10 | USC | Right | 62 | 23 | 7.54 | 54 |
| S11 | MRRI | Left | 60 | 14 | 6.17 | 39 |
| S12 | MRRI | Left | 71 | 13 | 13.58 | 49 |
| S13 | MRRI | Left | 52 | 12 | 4.33 | 50 |
| S14 | MRRI | Left | 75 | 13 | 4.92 | 51 |
| S15 | MRRI | Left | 67 | 12 | 1.08 | 63 |
| S16 | MRRI | Right | 52 | 13 | 5.33 | 34 |
| S17 | MRRI | Right | 68 | 14 | 8.17 | 47 |
| S18 | MRRI | Right | 56 | 13 | 1.5 | 49 |
| S19 | MRRI | Right | 64 | 12 | 7.17 | 41 |
| S20 | MRRI | Right | 62 | 16 | 2.92 | 50 |
| Mean (SD) | 62.5 (8.16) | 14.3 (2.68) | 5.8 (3.28) | 46 (8.27) |
Abbreviations: MRRI, Moss Rehabilitation Research Institute; USC, University of Southern California.
All participants were right-hand dominant.
Similarly, LCVA and RCVA patients were well matched in age, chronicity, education, and UEFM scores [t(18) < 1.2, P > .23 for all comparisons]. The MRRI and USC protocols were approved by the institutional review boards of Einstein Healthcare Network and USC, respectively. Participants at MRRI were paid for their participation in the study consistent with Einstein Healthcare Network policy.
Background Test Battery
Apraxia Task (Imitation of Novel Gestures).
To assess spatio-temporal deficits in imitation (a major hallmark of limb apraxia) without the confound of a tool knowledge component, we used our laboratory’s well-studied assessment of meaningless gesture imitation. Participants were shown videos of an experimenter performing 10 novel gestures while facing the viewer. The gestures were meaningless analogues of meaningful tool use gestures (see Buxbaum et al30 for details of stimulus development). There were 2 versions of the videos, and both LCVA and RCVA patients used their ipsilesional hand to copy the model in mirror perspective. Each gesture was shown twice in succession, and participants were permitted to begin imitating while observing. Participants’ gestures were videotaped and later scored using scoring criteria long in use at MRRI (see Buxbaum et al31). Both USC and MRRI patients’ performance was scored by trained coders in the Buxbaum lab who demonstrated reliability with previous coders in the lab, as defined by Cohen κ >0.85 (very good agreement32). Scores were converted to proportion of possible total points (maximum 100).
Spatial Neglect and Attention/Arousal Task.
To assess both lateralized and nonlateralized attention, participants performed the Virtual Reality Lateralized Attention Test (VRLAT), short form. In this game-like task, participants use a computer joystick to navigate down a winding path on a computer monitor. Images of a variety of static objects, including colored trees, animal statues, and road signs, are observable in quasirandomized locations on each side of the path; moving objects, including balls and skateboards, cross the path at random intervals, and there are random distracting noises. Participants’ task is to name the color of the trees and the animals depicted by the statues. The course is traversed once “coming” and once “going,” so the same objects appear once on each side of the path. Points are given for objects correctly named with a full description (“purple tree,” “cat statue”). The normalized difference in points awarded to the ipsilesional and contralesional sides (Ipsilesional — Contralesional/(Ipsilesional + Contralesional) is a measure of spatial neglect. Following from prior research (eg, Van Vleet and DeGutis33), total points irrespective of side (Ipsilesional + Contralesional) served as a measure of nonlateralized attention and arousal.34
Self-efficacy Questionnaire.
We used the 20-item Confidence in Arm and Hand Movement (CAHM) questionnaire to evaluate perceived self-efficacy in performing unimanual and bimanual functional tasks using the contralesional upper extremity in home and community contexts in patients with stroke (Lewthwaite, personal communication). The participants were asked about their level of confidence or certainty in performing a given task using their contralesional hand alone or in conjunction with the ipsilesional hand (eg, “How confident are you that with your [L/R] hand you can cut food with a knife and fork at a restaurant?”). Each item is scored from 0 to 100, with 100 indicating a very high level of confidence or certainty. Scores are averaged across the 20 items to render a total score between 0 and 100. In a previous study,10 the CAHM was found to be both valid and reliable (Cronbach α = 0.96; ICC = 0.91).
Actual Amount of Use Test
The Actual Amount of Use Test (AAUT) was developed by Taub, DeLuca, and Crago as an assessment of limb use.16,35,36,37 Sterr et al11,38 modified the AAUT to measure NU. Participants perform 14 upper-extremity tasks, first in a spontaneous condition—that is, without any instruction, supervision, or time limits—and next in a forced condition,38 wherein they are expressly instructed to use their contralesional hand. In this context, NU is defined as the difference between the ability to perform a task with the contralesional hand (in the forced condition) and the choice to use that hand (in the spontaneous condition). Video data are recorded in each condition. For the spontaneous condition, video recording is conducted unbeknownst to the participant, who is later debriefed at the end of the test.
Video data were analyzed post hoc by a trained observer in the Winstein Lab to assess 2 primary measures: amount of use (AOU) and quality of movement.†
The AOU is defined as the choice to use the contralesional hand and is coded as 0 or 1, where 0 indicates that the contralesional hand was not used. The sums of AOU scores for the 14 tasks was quantified for the spontaneous condition as well as for the forced condition. Thus, a maximum total of 14 points was possible for each condition. Whereas in most cases of individuals with mild paresis a full score of 14 on the forced condition would be expected, those with more moderate to severe paresis may not be able to complete the full complement of 14 tasks, thus scoring less than 14. NU was calculated as the relative difference between the sum of scores of AOU for the spontaneous and forced conditions (normalized for performance in the forced condition):
Given that the spontaneous condition assesses free choice and the forced condition assesses motor capacity (ie, what can be done), the normalized difference between the two, that is, NU, provides a metric of choice within the limits of the individual’s capacity. NU ranges from 0 to 1, where a score of 0 would indicate no NU, a score of 0.5 would indicate NU for half of the items of which an individual is capable of performing when the less-affected arm is constrained, and a score of 1 would indicate full NU for all items of which an individual is capable.
Data Analysis Approach
Prior to statistical analyses, all data were inspected to ensure that they met normality assumptions, and any skewed distributions were appropriately transformed. The distribution of education values was skewed (positively) and was, therefore, square root transformed. All other distributions met assumptions for parametric analyses.
Our main dependent variable was NU. We assessed whether NU differed as a function of hemisphere of stroke using 2-sample t tests. We determined the individual relationships of our predictors (demographic predictors, apraxia, attention/arousal, neglect, and self-efficacy) to NU with Pearson correlations. Any and all of the predictors demonstrating a correlation with NU at a lenient significance threshold (ie, P < .05 uncorrected for multiple comparisons) were entered into a multiple regression model with NU as the dependent measure. We assessed whether there was multicollinearity in the NU model by examining the Variance Inflation Factor for each independent variable. We then implemented a backward stepwise regression procedure wherein we used an r2 change test to compare the improvement in fit of the full model versus a model in which the least predictive variable was removed.
Results
Table 2 provides participants’ scores on the AAUT and the other indices administered.
Table 2.
AAUT and Background Test Performance.
| Participant | sAOU | fAOU | NU | VRLAT: Attention/Arousal |
VRLAT: Neglect | CAHM | Limb Apraxia |
|---|---|---|---|---|---|---|---|
| S1 | 2 | 10 | 0.80 | 0.98 | −0.03 | 36.3 | 0.95 |
| S2 | 3 | 11 | 0.73 | 0.95 | 0 | 21.3 | 0.90 |
| S3 | 6 | 14 | 0.57 | 0.9 | 0 | 53.0 | 0.72 |
| S4 | 3 | 13 | 0.77 | 0.93 | 0.03 | 43.0 | 0.82 |
| S5 | 2 | 13 | 0.85 | 0.95 | −0.05 | 74.0 | 0.90 |
| S6 | 4 | 14 | 0.71 | 0.9 | 0 | 61.8 | 0.77 |
| S7 | 7 | 14 | 0.50 | 0.98 | 0.03 | 59.5 | 0.80 |
| S8 | 6 | 14 | 0.57 | 0.98 | 0.03 | 73.0 | 0.90 |
| S9 | 10 | 14 | 0.29 | 1 | 0 | 90.5 | 0.87 |
| S10 | 6 | 14 | 0.57 | 1 | 0 | 48.0 | 0.77 |
| S11 | 4 | 14 | 0.71 | 0.98 | −0.03 | 43.1 | 0.77 |
| S12 | 9 | 14 | 0.36 | 0.93 | 0.08 | 54.5 | 0.75 |
| S13 | 3 | 12 | 0.75 | 0.75 | −0.13 | 36.0 | 0.80 |
| S14 | 2 | 13 | 0.85 | 0.88 | 0.03 | 52.5 | 0.87 |
| S15 | 6 | 14 | 0.57 | 0.88 | −0.09 | 81.5 | 0.85 |
| S16 | 2 | 9 | 0.78 | 0.85 | 0 | 36.5 | 0.82 |
| S17 | 3 | 12 | 0.75 | 0.88 | 0.03 | 54.5 | 0.82 |
| S18 | 11 | 14 | 0.21 | 1 | 0 | 74.5 | 0.90 |
| S19 | 3 | 11 | 0.73 | 0.93 | 0.03 | 40.0 | 0.82 |
| S20 | 6 | 14 | 0.57 | 1 | 0 | 64.3 | 0.90 |
Abbreviations: AAUT, Actual Amount of Use Test; CAHM, Confidence in Arm and Hand Movement; fAOU, forced amount of use; NU, nonuse; sAOU, spontaneous amount of use; VRLAT, Virtual Reality Lateralized Attention Test.
Individual Predictors of Nonuse
Demographic and Sensory-Motor Predictors.
NU scores did not differ in left- versus right-hemisphere stroke: t(18) = 0.59; P = .56. Furthermore, NU did not correlate with age (r = −0.11; P = .64), chronicity (r = 0.05; P = .83), or education (r = 0.02; P = .92).
Table 3 shows that all AAUT indices were significantly correlated with each other (all P values < .05). All the AAUT indices also correlated with the UEFM (P values < .01).
Table 3.
Correlations Between AAUT Indices and Background Measures.a
| Test/Index | UEFM | sAOU | fAOU | CAHM | VRLAT Total |
|---|---|---|---|---|---|
| UEFM | 1 | ||||
| sAOU | 0.58** | 1 | |||
| fAOU | 0.71*** | 0.71*** | 1 | ||
| CAHM | 0.63*** | 0.61*** | 0.67*** | 1 | |
| VRLAT total | −0.08 | 0.47* | 0.38 | 0.32 | 1 |
| NU | −0.54* | −0.99*** | −0.62*** | −0.55** | −0.45* |
Abbreviations: AAUT, Actual Amount of Use Test; CAHM, Confidence in Arm and Hand Movement; fAOU, forced amount of use; NU, nonuse; sAOU, spontaneous amount of use; UEFM, Upper-Extremity Fugl-Meyer; VRLAT, Virtual Reality Lateralized Attention Test.
*P < .05; **P < .01; ***P < .005.
Although arm sensory-motor impairment is likely to substantially drive these correlations, the upper- and lower-right quadrants of Figure 1 show that 65% (13/20) of participants have UEFM scores > 45 (corresponding to relatively mild impairment; see29,39) but nevertheless show some degree of NU (ie, NU score > 0), with at least 45% (9/20) demonstrating NU > 50% of the time (ie, score > 0.5). The data thus far suggest that factors other than sensory-motor impairment may influence NU behavior.
Figure 1.
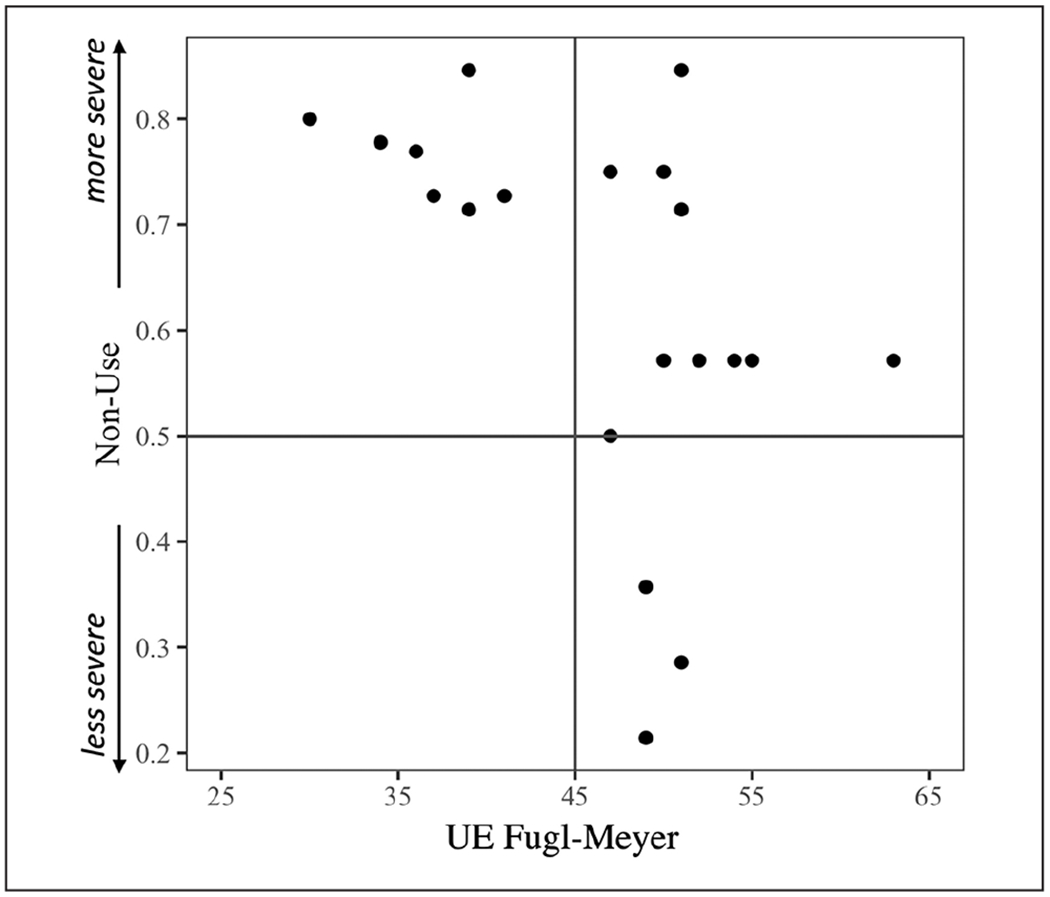
Relationship between nonuse (NU) in the Actual Amount of Use Test and Upper Extremity (UE) Fugl-Meyer. NU ranges from 0 to 1, where a score of 0 indicates no NU, 0.5 indicates NU for half of the items of which an individual is capable, and 1 indicates NU for all items of which they are capable. Figure quadrants are defined by median possible scores.
Psychological and Neuropsychological Predictors of Nonuse.
CAHM was negatively correlated with NU: r = −0.55; P = .01. Thus, greater CAHM was associated with reduced NU. Lower total scores on the VRLAT (attention/arousal) were associated with increased NU (r = −0.45; P = .04). The normalized difference score from the VRLAT (spatial neglect) was not correlated with NU (r = 0.15; P = .52).
Combined Predictive Models of AAUT Indices
Results of the backward stepwise regressions predicting NU with the independent variables UEFM, CAHM, and VRLAT Total are shown in Table 4. The full model (ie, including all 3 independent variables) significantly predicted NU, as would be expected given that each independent variable is correlated with NU. The Variance Inflation Factor score for each of the 3 independent variables was <3, indicating that our results were not influenced by multicollinearity.40
Table 4.
Results of Stepwise Regression Models Predicting Nonuse.
| Model | Predictors | β | Model r2 | r2 Δ From Full Model | P for r2 Δ |
|---|---|---|---|---|---|
| Full | FM | −0.01a | |||
| CAHM | −0.0007 | 0.49 | |||
| VRLAT Total | −1.5a | ||||
| CAHM removed | FM | −0.01a | 0.53 | 0.04 | .78 |
| VRLAT Total | −1.6a | ||||
| VRLAT removed | FM | −0.01a | 0.36 | 0.13 | .03a |
| CAHM | −0.004 |
Abbreviations: CAHM, Confidence in Arm and Hand Movement; FM, upper extremity Fugl-Meyer score; NU, nonuse; VRLAT, Virtual Reality Lateralized Attention Test.
P < .05.
The model with just UEFM and VRLAT total included (ie, with CAHM removed) accounted for as much variance in predicting NU as the full model with CAHM included. Importantly, however, removal of the VRLAT resulted in a more poorly fitting model as compared with the models with the VRLAT included. Thus, the preferred model for predicting NU contains UEFM and VRLAT total.
Post hoc Analysis
A post hoc correlational analysis was performed on those participants with UEFM >45 (n = 13; see Table 1) to assess whether there might be preliminary evidence for a different relationship between our predictors (UEFM, VRLAT Total, VRLAT Neglect score, Meaningless Imitation) and NU in this relatively mild group compared with those observed in the sample as a whole. Table 5 shows the significant results. In this relatively mild subset of the participant group, CAHM and VRLAT Total scores both correlated moderately strongly with NU.
Table 5.
Correlation Between NU and Background Measures (FM > 45; n = 13).
Abbreviations: CAHM, Confidence in Arm and Hand Movement; FM, upper extremity Fugl-Meyer score; NU, nonuse; VRLAT, Virtual Reality Lateralized Attention Test.
P < .05.
Discussion
This study provides preliminary evidence relevant to some of the demographic, sensory-motor, and other behavioral factors (apraxia, neglect, attention/arousal, and self-efficacy) that may predict arm NU in a sample of 20 chronic stroke survivors, half of whom had left- and half, right-hemisphere lesions. Because of our relatively small sample size, the study’s null results, in particular, should be viewed with caution. Despite this limitation, we demonstrated that 65% of patients with relatively mild impairment on the UEFM (ie, score > 45/66)29,39 nevertheless showed some degree of NU as measured by the AAUT (upper- and lower-right quadrants of Figure 1), with at least 45% of all participants demonstrating substantial NU (ie, >50% of the time), broadly consistent with prior reports of NU frequency.7 Thus, these relatively mildly impaired individuals have the capacity to use the arm and hand, but they choose not to use it at a level that capacity alone does not predict.
We also demonstrated that scores on a common measure of sensory-motor impairment, the UEFM, predicted the degree of NU. These data are broadly consistent with our earlier work demonstrating that arm and hand function measured immediately after therapy predicts, on average, the long-term change in arm use.41 In this prior research, we demonstrated that above a functional threshold, use improves over the long term. Below this threshold, use decreases. Consistent with the idea of a threshold for use, one recent study suggests that the relationship between motor impairment and NU might be better explained by a nonlinear function.42 In our study, the disproportionate tendency of individuals with UEFM <45 to exhibit NU (upper left quadrant of Figure 1) suggests that a functional threshold could potentially be used to identify patients who are likely to exhibit NU.
In this cohort, stroke survivors with greater sensory-motor impairment (ie, those with relatively less capacity) showed a larger relative disparity between their arm use in the forced use condition (when instructed to use the paretic side) and the spontaneous use condition (when they chose to use the paretic side), consistent with prior observations.7,8 In other words, the disparity between capability and use is not monotonic across the distribution of UEFM scores. For individuals with UEFM <45 (Figure 1, upper- and lower-left quadrants), NU is particularly pronounced (NU scores for all 7 participants were ≥0.7). Importantly, extending prior observations, we demonstrated that nonlateralized attention/arousal predicted NU above and beyond sensory-motor impairment. We will discuss these results in turn.
Attention/Arousal as a Predictor of Nonuse
The observed relationship between nonlateralized attention and NU is consistent with prior observations regarding the relationship of attention, effort, and motivation. That is, there is evidence that attention, which is often regarded as a physiological measure, may be an important prerequisite for effort and motivation, which are frequently viewed as relatively more “psychological” constructs. Nonlateralized attention/arousal was classically described as relying on ascending pathways originating from the midbrain reticular formation.43 More recent research has identified multiple circuits critical to attention and arousal, involving thalamic nuclei, hypothalamus, limbic regions, cingulate, frontal operculum, and basal ganglia. Accordingly, even small subcortical strokes may be associated with deficits in vigilance and attention.44 Ascending pathways through subcortical structures are modulated by a number of descending inputs from cortical regions, including the frontal and parietal cortex.45 Deficits in nonlateralized attention, though potentially more common after right-hemisphere stroke (eg, Heilman and Van Den Abell46) are observed in strokes affecting either hemisphere (eg, van Kessel et al47). Recently, Rinne et al48 demonstrated in both neurotypical aged and hemiparetic stroke participants a relationship between motor performance (dexterity and strength) and attention control, as measured by distractor resistance, even controlling for lesion size and baseline performance.
Previous investigations suggest that nonlateralized attention/arousal bears a relationship to mental effort and to goal-driven aspects of behavior. For example, arousal/effort as measured by pupil dilation appears necessary to direct eye gaze toward objects that are not salient but nevertheless relevant to current goals. Conversely, low arousal/effort as assessed by pupil constriction is associated with attentional capture by highly salient but irrelevant stimuli.49 An even more direct link between attention and goal-driven behavior is suggested by studies showing that individuals who demonstrate greater and more efficient sustained attention in laboratory tasks achieve high scores on scales of grit—the ability to persevere toward long-term goals.50,51
It has been argued that use of a paretic arm requires effort and motivation.17 Although the relationship between effort, motivation, attention, and NU in the long-term has not (to our knowledge) been directly assessed, several recently completed studies have assessed whether targeting attention and effort during training (eg, through the incorporation of games) may improve short-term outcomes. For example, a recent study showed that manual strength and hand function improved to a greater degree in a group of 25 participants who performed game-based exercises as compared with a group of 25 who performed time-matched traditional manual exercise, and motivation was self-rated as significantly greater in the former group.52 The successful Queen’s Square Program53 includes motivating computer games, self-efficacy, and goal setting in addition to intensive active practice. Focus groups conducted after the program indicated that patients believed that individualized goals and motivation were key ingredients in the program’s success. Finally, our group recently reported results of a planned intention-to-treat analysis of a large-scale study comparing an accelerated skill acquisition program (ASAP) that included motivational enhancements, autonomy support, and critical elements of the transfer package (eg, promotion of self-efficacy) with dose-matched usual therapy. Importantly, we demonstrated that the ASAP intervention substantially accelerated improvements across a spectrum of patient-reported outcomes that included physical function, reintegration into normal living, and health-related quality of life and exacted lasting gains in patient-reported overall strength.54
In the present study, a post hoc analysis of a subgroup of 13 patients with UEFM >45 (ie, patients with relatively mild sensory-motor impairment29,39) showed that our measure of attention/arousal correlated with UEFM and that this correlation was numerically larger (r = −0.65) than that observed in the study cohort as a whole (r = −0.45). Given the sample size, there was insufficient power to assess whether the correlation in the subsample was stronger than in the study cohort as a whole. The data are nevertheless consistent with the possibility that attention/arousal may be a particularly relevant predictor of NU in patients who have relatively mild sensory-motor dysfunction.‡. This remains an interesting question for future research.
Self-efficacy as a Predictor of Nonuse
The individual correlations we performed suggested that greater CAHM was associated with reduced NU. These relationships were weakened when we considered sensory-motor impairment in the same model, reflecting the shared variance between perceived confidence in movement and sensory-motor impairment. In other words, patients’ perceived confidence in what they could achieve in the future was realistically influenced by their abilities, and the latter was a relatively robust predictor of NU.
Recent evidence suggests that reduced self-efficacy may be associated with poorer rehabilitation outcomes. For example, a recent study with 120 patients showed that initial values of the Generalized Self-Efficacy Scale were correlated with scores on the Barthel Index and Rivermead Mobility Index (and other measures) performed after 3 weeks of daily rehabilitation treatment.55 Following from such data, recent approaches to stroke rehabilitation have increasingly emphasized the importance of interventions targeting self-efficacy. Sit et al,56 for example, performed a 2-arm, single-blind randomized controlled trial of 210 stroke patients and demonstrated that an “empowerment” intervention that included goal setting and action planning improved outpatient rehabilitation outcomes when compared with treatment without the added intervention (see also Marks et al57). Indeed, a recent phase IIb randomized controlled trial of therapy dose in chronic stroke survivors that used the ASAP intervention demonstrated a significant effect of dose on a patient-reported outcome measure of arm use (ie, Motor Activity Log-Quality of Movement), supporting the importance of perceived self-efficacy for effecting gains in spontaneous arm use in the natural environment.58,59
Study Limitations
Contrary to our expectation, we failed to observe a relationship between either limb apraxia or spatial neglect and limb NU; nor did we observe a relationship between variables such as age or hemisphere of stroke that might plausibly contribute. Because of the small sample size, caution should be exercised in interpreting these and any other null results. In addition, our selection criteria, which required UEFM scores in the range that corresponds to mild to moderate impairment, may have reduced our ability to observe some of these relationships. Specifically, because both apraxia and neglect severity tend to covary with overall stroke severity, our selection criteria appear to have resulted in exclusion of patients with moderate to severe neglect or limb apraxia. Given that (as noted earlier) both disorders are associated with poor rehabilitation outcomes, it remains possible that they may contribute to the prediction of limb NU in more severely impaired patients. Additional studies with a larger sample and broader range of patient severity will be necessary to clarify these relationships.
The sample in this preliminary study was limited in several other potentially important respects. No information was available about co-occurring sensory and motor impairments that may influence spontaneous arm use, such as peripheral neuropathy, sensory loss, spasticity, or joint mobility limitations. We also did not have information about whether stroke was of ischemic, hemorrhagic, or embolic origin; stroke type has been shown to influence outcomes overall, and it is unclear whether it may affect NU.60 The status of the “unimpaired” ipsilesional limb was also not assessed, and pain or movement limitations of that limb may plausibly influence use of the impaired limb. Moreover, given the small sample, we could not assess the interaction of hemisphere of stroke and hand dominance (concordance). Additionally, we did not assess overall cognitive function, pain, or mental health. Pain of the contralesional limbs has been hypothesized to be associated with NU.1 In terms of mental health, depression, in particular, has been negatively associated with rehabilitation outcome (eg, Hadidi et al61). Of interest for future research is the question of whether depression may mediate the relationships we observed between attention/arousal and NU, and confidence in movement and NU.
Conclusion
In this preliminary study, we demonstrated that a neuropsychological factor—attention/arousal—contributes to the prediction of upper-extremity NU in chronic stroke survivors with mild to moderate sensory-motor impairments. Given the complexity of the NU phenomenon, it follows that a combination of impairment and attention/arousal predicted NU behavior. The data support the importance of motivated engagement to drive attention to and use of the paretic limb. Although the relationship between motivation and attention during practice, and long-term predilection to spontaneously use the impaired limb is uncertain, it is increasingly clear that rehabilitation efforts are more likely to be successful when they engage the participant by providing meaningful task practice and motivational enhancements (see Winstein and Varghese62 for discussion). Larger-scale studies of the NU phenomenon in individuals ranging in severity and with incorporation of a number of additional behavioral (eg, vision, sensation, proprioception, spasticity, joint limitations, pain, mental health, motivation) and neuroanatomical measures (eg, lesion volume and white matter connectivity) will be required to extend these findings.
Acknowledgments
We appreciate the participation of the individuals whose data are included in this study.
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported by the Moss Rehabilitation Research Institute and the Division of Biokinesiology and Physical Therapy at the University of Southern California.
Footnotes
Declaration of Conflicting Interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
While these constructs are not synonymous, recent research suggests that there is a fundamental relationship between them.50,51
Quality of movement is outside of the scope of this manuscript and will not be discussed here.
We thank an anonymous reviewer for this suggestion.
References
- 1.Raghavan P Upper limb motor impairment after stroke. Phys Med Rehabil Clin N Am. 2015;26:599–610. doi: 10.1016/j.pmr.2015.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Taub E, Uswatte G, Mark VW, Morris DM. The learned nonuse phenonmenon: implications for rehabilitation. Eura Medicophys. 2006;42:241–256. [PubMed] [Google Scholar]
- 3.Wolf SL. Revisiting constraint-induced movement therapy: are we too smitten with the mitten? Is all nonuse “learned?” and other quandaries. Phys Ther. 2007;87:1212–1223. doi: 10.2522/ptj.20060355 [DOI] [PubMed] [Google Scholar]
- 4.Waddell KJ, Strube MJ, Bailey RR, et al. Does task-specific training improve upper limb performance in daily life post-stroke? Neurorehabil Neural Repair. 2017;31:290–300. doi: 10.1177/1545968316680493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Young NL, Williams JI, Yoshida KK, Bombardier C, Wright JG. The context of measuring disability: does it matter whether capability or performance is measured? J Clin Epidemiol. 1996;49:1097–1101. doi: 10.1016/0895-4356(96)00214-4 [DOI] [PubMed] [Google Scholar]
- 6.Bailey RR, Klaesner JW, Lang CE. Quantifying real-world upper-limb activity in nondisabled adults and adults with chronic stroke. Neurorehabil Neural Repair. 2015;29:969–978. doi: 10.1177/1545968315583720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bakhti KKA, Mottet D, Schweighofer N, Froger J, Laffont I. Proximal arm non-use when reaching after a stroke. Neurosci Lett. 2017;657:91–96. doi: 10.1016/j.neulet.2017.07.055 [DOI] [PubMed] [Google Scholar]
- 8.Stewart JC, Cramer SC. Patient-reported measures provide unique insights into motor function after stroke. Stroke. 2013;44:1111–1116. doi: 10.1161/STROKEAHA.111.674671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van der Lee JH, Wagenaar RC, Lankhorst GJ, Vogelaar TW, Deville WL, Bouter LM. Forced use of the upper extremity in chronic stroke patients: results from a single-blind randomized clinical trial. Stroke. 1999;30:2369–2375. [DOI] [PubMed] [Google Scholar]
- 10.Wolf SL, Winstein CJ, Miller JP, et al. Effect of constraint-induced movement therapy on upper extremity function 3 to 9 months after stroke: the EXCITE randomized clinical trial. JAMA. 2006;296:2095–2104. doi: 10.1001/jama.296.17.2095 [DOI] [PubMed] [Google Scholar]
- 11.Sterr A, Elbert T, Berthold I, Koölbel S, Rockstroh B, Taub E. Longer versus shorter daily constraint-induced movement therapy of chronic hemiparesis: an exploratory study. Arch Phys Med Rehabil. 2002;83:1374–1377. doi: 10.1053/apmr.2002.35108 [DOI] [PubMed] [Google Scholar]
- 12.Park J, Lee N, Cho Y, Yang Y. Modified constraint-induced movement therapy for clients with chronic stroke: interrupted time series (ITS) design. J Phys Ther Sci. 2015;27:963–966. doi: 10.1589/jpts.27.963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Takebayashi T, Koyama T, Amano S, et al. A 6-month follow-up after constraint-induced movement therapy with and without transfer package for patients with hemiparesis after stroke: a pilot quasi-randomized controlled trial. Clin Rehabil. 2013;27:418–426. doi: 10.1177/0269215512460779 [DOI] [PubMed] [Google Scholar]
- 14.Ballester BR, Maier M, San Segundo Mozo RM, Castada V, Duff A, Verschure PFMJ. Counteracting learned non-use in chronic stroke patients with reinforcement-induced movement therapy. J Neuroeng Rehabil. 2016;13:74. doi: 10.1186/s12984-016-0178-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu XH, Huai J, Gao J, Zhang Y, Yue SW. Constraint-induced movement therapy in treatment of acute and sub-acute stroke: a meta-analysis of 16 randomized controlled trials. Neural Regen Res. 2017;12:1443–1450. doi: 10.4103/1673-5374.215255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taub E, Uswatte G, Pidikiti R. Constraint-induced movement therapy: a new family of techniques with broad application to physical rehabilitation—a clinical review. J Rehabil Res Dev. 1999;36:237–251. [PubMed] [Google Scholar]
- 17.Sunderland A, Tuke A. Neuroplasticity, learning and recovery after stroke: a critical evaluation of constraint-induced therapy. Neuropsychol Rehabil. 2005;15:81–96. doi: 10.1080/09602010443000047 [DOI] [PubMed] [Google Scholar]
- 18.Wulf G, Lewthwaite R. Optimizing performance through intrinsic motivation and attention for learning: the OPTIMAL theory of motor learning. Psychon Bull Rev. 2016;23:1382–1414. doi: 10.3758/s13423-015-0999-9 [DOI] [PubMed] [Google Scholar]
- 19.Taub E, Uswatte G, Mark VW, et al. Method for enhanceing real-world use of a more affected arm in chronic stroke: transfer package of constraint-induced movement therapy. Stroke. 2013;44:1383–1388. doi: 10.1161/STROKEAHA.111.000559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gauthier LV, Taub E, Perkins C, Ortmann M, Mark VW, Uswatte G. Remodeling the brain: plastic structural brain changes produced by different motor therapies after stroke. Stroke. 2008;39:1520–1525. doi: 10.1161/STROKEAHA.107.502229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Katz N, Hartman-Maeir A, Ring H, Soroker N. Functional disability and rehabilitation outcome in right hemisphere damaged patients with and without unilateral spatial neglect. Arch Phys Med Rehabil. 1999;80:379–384. [DOI] [PubMed] [Google Scholar]
- 22.van Dalen JW, van Charante EPM, Nederkoorn PJ, van Gool WA, Richard E. Poststroke apathy. Stroke. 2013;44:851–860. doi: 10.1161/STROKEAHA.112.674614 [DOI] [PubMed] [Google Scholar]
- 23.Sundet K, Finset A, Reinvang I. Neuropsychological predictors in stroke rehabilitation. J Clin Exp Neuropsychol. 1988;10:363–379. doi: 10.1080/01688638808408245 [DOI] [PubMed] [Google Scholar]
- 24.Chen S, Lewthwaite R, Schweighofer N, Winstein CJ. Discriminant validity of a new measure of self-efficacy for reaching movements after stroke-induced hemiparesis. J Hand Ther. 2013;26:116–123. doi: 10.1016/j.jht.2012.09.002 [DOI] [PubMed] [Google Scholar]
- 25.Sugg K, Müller S, Winstein C, Hathorn D, Dempsey A. Does action observation training with immediate physical practice improve hemiparetic upper-limb function in chronic stroke? Neurorehabil Neural Repair. 2015;29:807–817. doi: 10.1177/1545968314565512 [DOI] [PubMed] [Google Scholar]
- 26.Stewart JC, Lewthwaite R, Rocktashel J, Winstein CJ. Self-efficacy and reach performance in individuals with mild motor impairment due to stroke. Neurorehabil Neural Repair. 2019;33:319–328. doi: 10.1177/1545968319836231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schwartz MF, Brecher AR, Whyte J, Klein MG. A patient registry for cognitive rehabilitation research: a strategy for balancing patients’ privacy rights with researchers’ need for access. Arch Phys Med Rehabil. 2005;86:1807–1814. doi: 10.1016/j.apmr.2005.03.009 [DOI] [PubMed] [Google Scholar]
- 28.Woytowicz EJ, Westlake KP, Whitall J, Sainburg RL. Handedness results from complementary hemispheric dominance, not global hemispheric dominance: evidence from mechanically coupled bilateral movements. J Neurophysiol. 2018;120:729–740. doi: 10.1152/jn.00878.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Woodbury ML, Velozo CA, Richards LG, Duncan PW. Rasch analysis staging methodology to classify upper extremity movement impairment after stroke. Arch Phys Med Rehabil. 2013;94:1527–1533. doi: 10.1016/j.apmr.2013.03.007 [DOI] [PubMed] [Google Scholar]
- 30.Buxbaum LJ, Kyle KM, Menon R. On beyond mirror neurons: internal representations subserving imitation and recognition of skilled object-related actions in humans. Brain Res Cogn Brain Res. 2005;25:226–239. doi: 10.1016/j.cog-brainres.2005.05.014 [DOI] [PubMed] [Google Scholar]
- 31.Buxbaum LJ, Shapiro AD, Coslett HB. Critical brain regions for tool-related and imitative actions: a componential analysis. Brain. 2014;137(pt 7):1971–1985. doi: 10.1093/brain/awu111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Altman DG. Statistics in medical journals: developments in the 1980s. Stat Med. 1991;10:1897–1913. doi: 10.1002/sim.4780101206 [DOI] [PubMed] [Google Scholar]
- 33.Van Vleet TM, DeGutis JM. The nonspatial side of spatial neglect and related approaches to treatment. Prog Brain Res. 2013;207:327–349. doi: 10.1016/B978-0-444-63327-9.00012-6 [DOI] [PubMed] [Google Scholar]
- 34.Buxbaum LJ, Dawson AM, Linsley D. Reliability and validity of the Virtual Reality Lateralized Attention Test in assessing hemispatial neglect in right-hemisphere stroke. Neuropsychology. 2012;26:430–441. doi: 10.1037/a0028674 [DOI] [PubMed] [Google Scholar]
- 35.Taub E, DeLuca S and Crago JE. Actual Amount of Use Test. Unpublished Manuscript Birmingham, AL: Psychology Department, University of Alabama, 1996. [Google Scholar]
- 36.Kunkel A, Kopp B, Müller G, et al. Constraint-induced movement therapy for motor recovery in chronic stroke patients. Arch Phys Med Rehabil. 1999;80:624–628. doi: 10.1016/S0003-9993(99)90163-6 [DOI] [PubMed] [Google Scholar]
- 37.Uswatte G, Taub E. Constraint-induced movement therapy: new approaches to outcome measurement in rehabilitation. In: Stuss DT, Winocur G, Robertson IH, eds. Cognitive Neurorehabilitation. New York, NY: Cambridge University Press; 1999:215–229. [Google Scholar]
- 38.Sterr A, Freivogel S, Schmalohr D. Neurobehavioral aspects of recovery: assessment of the learned nonuse phenomenon in hemiparetic adolescents. Arch Phys Med Rehabil. 2002;83:1726–1731. [DOI] [PubMed] [Google Scholar]
- 39.Woytowicz EJ, Rietschel JC, Goodman RN, et al. Determining levels of upper extremity movement impairment by applying a cluster analysis to the Fugl-Meyer assessment of the upper extremity in chronic stroke. Arch Phys Med Rehabil. 2017;98:456–462. doi: 10.1016/j.apmr.2016.06.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kutner MH, Nachtsheim CJ, Neter J, Li W. Applied Linear Statistical Models. 5th ed. New York, NY: McGraw-Hill; 2005. [Google Scholar]
- 41.Schweighofer N, Han CE, Wolf SL, Arbib MA, Winstein CJ. A functional threshold for long-term use of hand and arm function can be determined: predictions from a computational model and supporting data from the Extremity Constraint-Induced Therapy Evaluation (EXCITE) Trial. Phys Ther. 2009;89:1327–1336. doi: 10.2522/ptj.20080402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rafiei MH, Kelly KM, Borstad AL, Adeli H, Gauthier LV. Predicting improved daily use of the more affected arm post-stroke following constraint-induced movement therapy. Phys Ther. 2019;99:1667–1678. doi: 10.1093/ptj/pzz121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moruzzi G, Magoun HW. Brain stem reticular formation and activation of the EEG. Electroencephalogr Clin Neurophysiol. 1949;1:455–473. [PubMed] [Google Scholar]
- 44.Malik PRA, Muir RT, Black SE, et al. Subcortical brain involvement is associated with impaired performance on the psychomotor vigilance task after minor stroke. Neurorehabil Neural Repair. 2018;32:999–1007. doi: 10.1177/1545968318804415 [DOI] [PubMed] [Google Scholar]
- 45.Boukrina O, Barrett AM. Disruption of the ascending arousal system and cortical attention networks in post-stroke delirium and spatial neglect. Neurosci Biobehav Rev. 2017;83:1–10. doi: 10.1016/j.neubiorev.2017.09.024 [DOI] [PubMed] [Google Scholar]
- 46.Heilman KM, Van Den Abell T. Right hemisphere dominance for attention: the mechanism underlying hemispheric asymmetries of inattention (neglect). Neurology. 1980;30:327–330. doi: 10.1212/wnl.30.3.327 [DOI] [PubMed] [Google Scholar]
- 47.van Kessel ME, van Nes IJW, Brouwer WH, Geurts ACH, Fasotti L. Visuospatial asymmetry and non-spatial attention in subacute stroke patients with and without neglect. Cortex. 2010;46:602–612. doi: 10.1016/j.cortex.2009.06.004 [DOI] [PubMed] [Google Scholar]
- 48.Rinne P, Hassan M, Fernandes C, et al. Motor dexterity and strength depend upon integrity of the attention-control system. Proc Natl Acad Sci U S A. 2017;115:E536–E545. doi: 10.1073/pnas.1715617115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mathôt S, Siebold A, Donk M, Vitu F. Large pupils predict goal-driven eye movements. J Exp Psychol Gen. 2015;144:513–521. doi: 10.1037/a0039168 [DOI] [PubMed] [Google Scholar]
- 50.Dimenichi BC, Richmond LL. Reflecting on past failures leads to increased perseverance and sustained attention. J Cogn Psychol. 2015;27:180–193. doi: 10.1080/20445911.2014.995104 [DOI] [Google Scholar]
- 51.Kalia V, Thomas R, Osowski K, Drew A. Staying alert? Neural correlates of the association between Grit and Attention Networks. Front Psychol. 2018;9:1377. doi: 10.3389/fpsyg.2018.01377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Park JS, Lee G, Choi JB, Hwang NK, Jung YJ. Game-based hand resistance exercise versus traditional manual hand exercises for improving hand strength, motor function, and compliance in stroke patients: a multi-center randomized controlled study. NeuroRehabilitation. 2019;45:221–227. doi: 10.3233/nre-192829 [DOI] [PubMed] [Google Scholar]
- 53.Ward NS, Brander F, Kelly K. Intensive upper limb neurorehabilitation in chronic stroke: outcomes from the Queen Square programme. J Neurol Neurosurg Psychiatry. 2019;90:498–506. doi: 10.1136/jnnp-2018-319954 [DOI] [PubMed] [Google Scholar]
- 54.Lewthwaite R, Winstein CJ, Lane CJ, et al. Accelerating stroke recovery: body structures and functions, activities, participation, and quality of life outcomes from a large rehabilitation trial. Neurorehabil Neural Repair. 2018;32:150–165. doi: 10.1177/1545968318760726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kobylańska M, Kowalska J, Neustein J, et al. The role of biopsychosocial factors in the rehabilitation process of individuals with a stroke. Work. 2019;61:523–535. doi: 10.3233/WOR-162823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sit JWH, Chair SY, Choi KC, et al. Do empowered stroke patients perform better at self-management and functional recovery after a stroke? A randomized controlled trial. Clin Interv Aging. 2016;11:1441–1450. doi: 10.2147/CIA.S109560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Marks R, Allegrante JP, Lorig K. A review and synthesis of research evidence for self-efficacy-enhancing interventions for reducing chronic disability: implications for health education practice (part II). Health Promot Pract. 2005;6:148–156. doi: 10.1177/1524839904266792 [DOI] [PubMed] [Google Scholar]
- 58.Winstein C, Lewthwaite R, Blanton SR, Wolf LB, Wishart L. Infusing motor learning research into neurorehabilitation practice: a historical perspective with case exemplar from the accelerated skill acquisition program. J Neurol Phys Ther. 2014;38:190–200. doi: 10.1097/NPT.0000000000000046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Winstein C, Kim B, Kim S, Martinez C, Schweighofer N. Dosage matters. Stroke. 2019;50:1831–1837. doi: 10.1161/STROKEAHA.118.023603 [DOI] [PubMed] [Google Scholar]
- 60.Dušica SP, Devečerski GV, Jovićević MN, Platiša NM. Stroke rehabilitation: which factors influence the outcome? Ann Indian Acad Neurol. 2015;18:484–487. doi: 10.4103/0972-2327.165480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hadidi N, Treat-Jacobson DJ, Lindquist R. Poststroke depression and functional outcome: a critical review of literature. Heart Lung. 2009;38:151–162. doi: 10.1016/j.hrtlng.2008.05.002 [DOI] [PubMed] [Google Scholar]
- 62.Winstein C, Varghese R. Been there, done that, so what’s next for arm and hand rehabilitation in stroke? NeuroRehabilitation. 2018;43:3–18. doi: 10.3233/NRE-172412 [DOI] [PubMed] [Google Scholar]


