Abstract
Introduction
Loss-to-follow-up among women living with HIV (WLWHIV) may lead to unfavorable outcomes for both mother and exposed infant. This study traced WLWHIV disengaged from care and their infants and compared their outcomes with those retained in care.
Methods
The study included WLWHIV who initiated ART during pregnancy at six public clinics in Uganda. A woman was defined as disengaged (DW) if she had not attended her 6-week post-partum visit by 10 weeks after her estimated date of delivery. DW were matched with retained women (RW) by age and duration on ART. Nurse counselors traced all selected DW via telephone and community visits to assess vital status, infant HIV sero-status and maternal HIV viral load through blood draws.
Results
Between July 2017 and July 2018, 734 women (359 DW and 375 RW) were identified for the study. Tracing was attempted on 349 DW and 160 (44.6%) were successfully located and enrolled in the study. They were matched with 162 RW. Among DW, 52 (32.5%) transferred to another health facility. Very few DW, 39.0% were HIV virally suppressed (<1000 copies/ml) compared to RW 89.5%, P<0.001). Among 138 babies born to DW, 4.3% tested positive for HIV compared to 1.4% among babies born to RW (P = 0.163).
Conclusion
Pregnant and breastfeeding WLWHIV who disengage from care are difficult to find in urban environments. Many have detectable viral loads, leading to the potential for an increased risk of MTCT. Efforts to reduce disengagement from care are critical for the successful elimination of MTCT in resource-limited settings.
Introduction
Provision of lifelong antiretroviral therapy (ART) to all pregnant women living with HIV, initially under the Option B+ policy and now under Treat All, has led to a 48% reduction in mother-to-child transmission (MTCT) in sub-Saharan Africa (SSA) [1,2], and has averted 1.4 million new HIV infections since 2010 [2]. In 2019, data from UNAIDS showed that 93% of pregnant women living with HIV in Eastern and Southern Africa received ART, resulting in low MTCT rates [3]. Despite these successes, in 2018, 180,000 children became infected with HIV globally [3], undermining the target of an HIV-free generation. MTCT is often the result of mothers disengaging from care following ART initiation [4–7], with infections occurring during the breastfeeding and postnatal periods rather than during pregnancy [8]. In 2019, 38 000 new infections occurred in UNAIDS focus African countries because women living with HIV did not receive antiretroviral therapy during pregnancy and 29 000 because women dropped out of antiretroviral therapy [9]. Disengagement from care may lead to unfavorable outcomes and true transmission rates are likely to be underestimated if women and infants who are lost to follow-up (LTFU) are not accounted for. HIV programs may underestimate transmission as women who are not in care may not be on ART, not virally suppressed, with higher risks of MTCT.
Several evaluations of country-specific prevention of mother-to-child transmission (PMTCT) programs have been performed in SSA, in countries such as Malawi [10,11], Nigeria and South Africa [12]. In Kenya, the PMTCT program led to a decline in maternal HIV transmission from 10.9% to 2.5% [13]. In Uganda, the percentage of children born to women living with HIV (WLWHIV) who become infected is estimated at 2.5% at 6 weeks post-partum and 5.3% at the end of the breastfeeding period [14]. Nevertheless, while most countries have achieved reductions in the rate of vertical HIV transmission, they have also reported high attrition among women initiating ART during pregnancy [15].
Studies conducted in Kenya and South Africa found that 76% of women adhered to ART during pregnancy, but only 53% returned for post-partum clinic appointments [16]. In Malawi, women initiating ART within PMTCT programs were five times more likely to drop out of care compared to non-pregnant women [17]. However, little is known about maternal and infant outcomes once a woman is LTFU. Understanding the impact of disengagement during pregnancy and the early post-partum period on maternal and infant outcomes is critical to assessing the efficacy of initiatives like Option B+ and Treat All, and for generating more accurate global estimates of MTCT of HIV.
Several tracing studies have addressed the outcomes of non-pregnant adults who have become LTFU [18–21]. However, other than three studies from Zimbabwe, eSwatini and Malawi, there is a dearth of knowledge about the outcomes of pregnant women initiating ART who subsequently become LTFU [22–24]. Such outcomes may include estimates of HIV viral suppression, MTCT and care status among women who disengage from HIV care programs. Given the paucity of data, this study aimed to trace women who disengaged from PMTCT services through community outreach. Our goals were to compare maternal and infant outcomes, such as vertical transmission and maternal HIV viral suppression, between women who were disengaged and those who were retained in care, and to identify the reasons for disengagement.
Methods
Ethical considerations
The study received approval from the Joint Clinical Research Center Institutional Review and Ethics Committee, the Uganda National Council of Science and Technology (Ref: HS35ES) and the Indiana University Institutional Review Board. All enrolled women provided written informed consent for study participation for themselves and their infants.
Study site and population
The study was conducted in Uganda at six public Kampala City Council Authority (KCCA) clinics supported by the Infectious Diseases Institute (IDI) and the Uganda Centers for Disease Control-President’s Emergency Plan for AIDS Relief (CDC-PEPFAR) HIV care program. Study activities leveraged the routine services offered at the PMTCT clinics for newly diagnosed pregnant WLWHIV, which included pre-ART adherence counseling, and same-day ART initiation of tenofovir+lamivudine+efavirenz (TDF+3TC+EFV), with a two-week return appointment. HIV viral load testing is done 6 months after ART initiation and yearly thereafter, as per Uganda National HIV Viral load testing algorithm. Women undertake monthly follow-up appointments during pregnancy and throughout the first 18 months of the post-partum period, consistent with the national Maternal Child Health (MCH) schedule for both women and HIV exposed infants [25,26]. WLWHIV 18 years of age or older, who initiated ART during pregnancy and were at least 6–12 weeks post-partum but less than 36 weeks were eligible for the study. The gestational age was estimated based on the date of the woman’s first antenatal clinic and database closure. Women known to have died or transferred out to another HIV care facility were excluded.
Study design
This was a prospective cohort study that enrolled women retained and disengaged from HIV care. A woman was considered disengaged (DW) if she had not had a clinic visit in the 90 days preceding database closure and had not returned for her scheduled 6-week postpartum visit by 10 weeks post-partum. The time point of the disengagement was assigned at 90 days after the woman’s last scheduled clinic appointment. The DW were further categorized into two groups; women who delivered while in care and those who disengaged before delivery. The estimated date of delivery (EDD) was used to determine the postpartum time frame for women who disengaged before delivery.
A woman was considered retained (RW) if she was actively attending the health care facility with at least one clinic encounter within 90 days of database closure and had attended her 6-week post-partum visit. All disengaged women were matched one-to-one with a retained woman by age (plus or minus 5 years) and months from ART initiation to study enrollment (plus or minus one month). Two retained women were erroneously included in the study prior to community tracing and enrollment of their matched disengaged women, causing an excess of RW. Age and duration on ART were selected for matching, as these are known to be associated with for disengagement from PMTCT programs [27]. Potential facility level factors associated with disengagement from care were well balanced since all the study sites serve similar catchement areas around Kampala city, and have similar governance and staffing structures.
Study procedures
Using information recorded in the Uganda Electronic Medical Record (UgandaEMR) [28] available at each of the study sites, all women who met the definition of DW or RW were flagged. All DW were first contacted through a telephone call to confirm personal details prior to community tracing and to ascertain their willingness to be traced. If uncontactable over the telephone, the study nurse counselor and one member from the health facility outreach team proceeded to the community using information recorded in the mother’s clinic file. During community outreach, confidentiality of a mother’s HIV status was maintained, and the study team identified themselves as representatives from the national MCH program. Blood samples were collected from the disengaged women from home or at the health facility, and their infants for HIV viral load and HIV DNA PCR testing, respectively. A viral load of <1000 copies/ml was considered undetectable. All DW who reported not being in care were encouraged to re-engage in care with their infant and were re-contacted one month after study enrollment to determine if they linked back to care and results of their laboratory testing provided to them during their next clinic appointment or via the telephone or a home visit if preferred.
For DW who reported that they had transferred to another HIV care and treatment facilities, part of the collected blood samples were used to determine drug levels of efavirenz using high performance liquid chromatography with ultra-violent detection. Drug levels were performed among disengaged women who reported to have transferred care, as way of validating their care status with the amount of drug levels. Retained women whose age and duration from ART initiation matched those of the DW who reported to have transferred to another facility were similarly tested for efavirenz drug levels.
Retained women were contacted via telephone, approached by the nurse counselor during their next clinic appointment and enrolled. Similarly, blood samples were collected from RW and their infants for HIV viral load and HIV DNA PCR testing, respectively. All infants were enrolled in the study if they were less than 9 months old. Results from the laboratory investigations were provided to the retained women approximately two weeks after study enrolment.
Data collection
The REDCap mobile application using Android-powered computer tablets was used for study data collection and storage. Information was collected using the UgandaEMR [28], and structured questionnaires. Information extracted from the UgandaEMR included dates of clinic registration and ART initiation plus clinical variables (e.g. weight, CD4 cell count, WHO clinical stage) at ART initiation and subsequent clinic encounters. The structured questionnaires collected information on infant HIV testing, breastfeeding and complimentary feeding practices, experience of intimate partner violence and disclosure of HIV status. Additional information on engagement in care status at the time of outreach and reasons for disengagement from care were collected from the DW.
Statistical analysis
We compared demographic and clinical characteristics between DW and RW at ART initiation and at study enrollment. We used chi-square tests for associations between two categorical variables and the Fisher’s Exact for small cell sizes (expected values less than 5). The Kruskal Wallis test was used to compare differences in continuous and ordinal variables between the two groups. All analyses were performed using the STATA software version 15.2 (Stata Corp, College Station, TX).
Results
Outcomes of retained and disengaged women
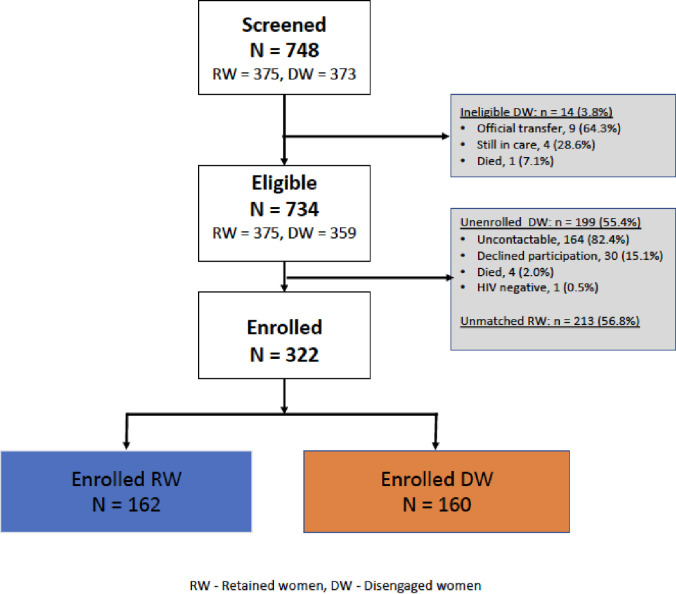
Between July 2017 and July 2018, a total of 748 WLWHIV were initiated on ART during pregnancy and enrolled in the PMTCT program at all the six KCCA clinics in Kampala, with 375 ultimately being retained and 373 being disengaged. Of these, 734 (375 RW and 359 DW) were eligible for the study (Fig 1). Among eligible RW, 213 (56.8%) were not matched with a DW and were not enrolled in the study. Of 199 DW (55.4%) who were not enrolled, 164 (82.4%) were uncontactable, 30 (15.1%) declined participation, 4 (2.0%) were found to be deceased and 1 (0.5%) woman subsequently tested HIV negative. Consequently, a total of 322 women (162 RW and 160 DW) were enrolled in the study (Fig 1). Retained and disengaged women had similar age, marital status and CD4 count at ART initiation, while RW had a slightly longer duration from ART initiation at study enrolment, median 9.5 months (IQR: 8.1,10.9) compared to 9.0 months (7.7, 10.5) among DW, P = 0.026 (Table 1). A larger proportion, 58.1%, of DW had a low education level (primary or none) compared to RW 37.7%, P<0.001, and 152 (95.0%) DW initiated ART later in their pregnancy (in the second or third trimester) as compared to 139 RW (85.8%; P = 0.005).
Fig 1. Study cohort diagram showing HIV positive retained and disengaged women enrolled in a community outreach study between July 2017 –July 2018 from six Kampala City Council public municipal clinics in Uganda.
Table 1. Demographic and clinical characteristics of retained and disengaged women at study enrollment.
| Characteristic | Retained Women (RW) (N = 162) | Disengaged Women (DW) (N = 160) | P Value |
|---|---|---|---|
| Age in years, Median (IQR) | 26.3 (23.6–29.1) | 25.6 (22.8–28.4) | 0.123 |
| Marital status1, n (%) | |||
| Single | 12 (9.8) | 8 (7.3) | 0.123 |
| Married/cohabiting | 87 (71.3) | 68 (62.4) | |
| Separated/divorced/widowed | 23 (18.9) | 33 (30.3) | |
| Education level, n (%) | |||
| None/primary | 61 (37.7) | 93 (58.1) | <0.001 |
| Secondary | 91 (56.2) | 61(38.1) | |
| Tertiary | 10 (6.2) | 6 (3.8) | |
| CD4 cells/uL at ART start2, Median (IQR) | 445 (319, 600) | 501 (324, 636) | 0.442 |
| Trimester at ART start, n (%) | |||
| I | 23 (14.2) | 8 (5.0) | 0.005 |
| II/III | 139 (85.8) | 152 (95.0) | |
| Duration in months between ART initiation and study enrollment, Median (IQR) | 9.5 (8.1,10.9) | 9.0 (7.7–10.5) | 0.026 |
Note
1Marital status collected among 122 RW and 109 DW.
2CD4 cell counts at ART initiation available for 119 RW and 79 DW, IQR interquartile range, ART Antiretroviral therapy.
Among the 160 DW enrolled in the study, 52 (32.5%) reported that they were receiving care at another health facility (self-transfers) (Table 2). The majority of DW (99; 71.7%) delivered after they had disengaged from care, while 39 (28.3%) delivered while in care (Table 2). Both groups of DW were similar with regard to age, education level, and marital status. Upon tracing, 24 (61.5%) women who delivered before disengagement and 69 (69.7%) who delivered after disengagement, had still not re-engaged in care anywhere else. Among the 22 DW who did not have babies enrolled, 11 (50.0%) delivered their infant or experienced the respective pregnancy outcome before disengaging from care.
Table 2. Maternal outcomes among retained and disengaged women in a PMTCT program in Uganda.
| Maternal Outcomes | Retained Women (RW) (N = 162) | Disengaged Women (DW) (N = 160) | P value |
|---|---|---|---|
| Timing of disengagement | |||
| Delivered before disengaged, n (%) | N/A | 39 (28.3)1 | N/A |
| HIV Viral load | |||
| VL < 1000 copies/ml, n (%) | 145 (89.5) | 62 (39.0)2 | <0.001 |
| Care status at successful outreach, n (%) | |||
| In care elsewhere (Transfers) | N/A | 52 (32.5) | N/A |
| Detectable Efavirenz concentrations3, n (%) | 45 (90.0) | 36 (72.0) | 0.022 |
| Post-partum family planning use, n (%) | 57 (35.2) | 36 (22.5) | 0.012 |
| Disclosed HIV status to anyone, n (%) | 109 (67.3) | 87 (54.4) | 0.018 |
| Disclosed HIV status to spouse4, n (%) | 75 (68.8) | 48 (55.2) | 0.050 |
1Out of 138 women with data on timing of delivery.
2One DW had missing HIV viral load result.
3Out of 50 DW reporting being in care and 50 matched RW.
4Among the 109 RW and 87 DW who had disclosed their status.
HIV viral suppression
Of women with viral load results (DW 159, RW 162), DW had significantly lower (39.0%) HIV viral suppression rates compared to RW (89.5%; P<0.001 (Table 2). Of the 52 DW who had self-transferred, 51 had HIV viral load results and a greater percentage of these, 70.6% (36/51), had achieved viral suppression compared to 24.1% (26/108) of those who were out of care (P<0.001).
Fifty of the DW who self-transferred and their matched RW were tested for detectable drug levels. Of the 50 DW, 36 (72.0%) had detectable efavirenz drug concentration. Among the 50 matched RW, 45 (90.0%) had detectable efavirenz drug concentrations, P = 0.022 (Table 2). HIV viral suppression was higher 78/81 (96.3%) among women with detectable efavirenz levels compared to 2/19 (10.5%) of women with no efavirenz concentrations, P<0.001.
HIV status disclosure and uptake of post-partum family planning
Among the 160 DW, 48 (55.2%) had disclosed their HIV status to their partner, compared to 75 of 162 RW (68.8%; P = 0.050) (Table 2). Among DW, 36 women (22.5%) were using any family planning method during the postpartum period compared to 57 RW (35.2%; P = 0.012) (Table 2).
Follow-up of disengaged women
An attempt was made to contact all disengaged women one month after study enrollment; at that time 10 (6.3%) were uncontactable. Despite initial contact and extensive counseling, 37 (24.7%) of the remaining 150 DW had not re-engaged in care. Reasons for not re-engaging were not mutually exclusive and included lack of transport to the health facility (41.6%), fear of pill burden (33.3%), denial of HIV status (12.8%), belief in spiritual healing (7.7%), HIV negative status of infant (2.6%) and use of herbal medicine to treat HIV (2.6%).
Outcomes of babies born to retained and disengaged women
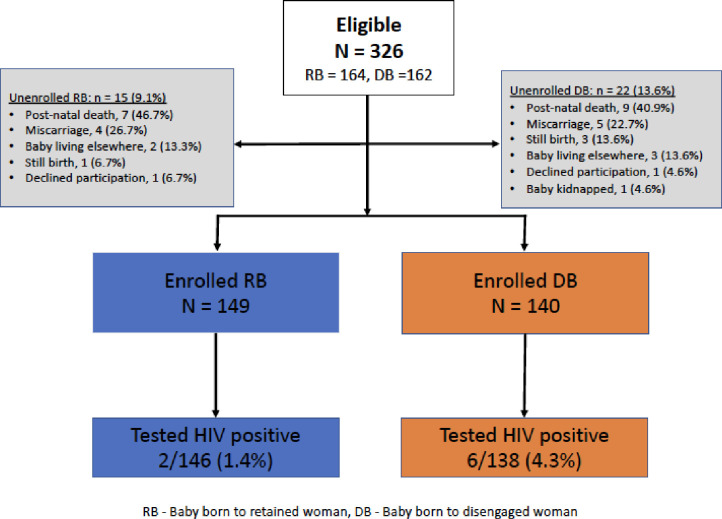
A total of 326 fetuses (164 RW and 162 DW) were observed among the 322 enrolled women, four pregnancies (2 DW and 2 RW) were twins. Among the 162 pregnancies in RW, 149 (90.9%) babies were alive and enrolled, 3 (1.8%) were not enrolled, while 12 (7.3%) pregnancies had adverse outcomes (7 post-natal deaths, 1 still birth and 4 miscarriages). Among the 3 infants who were not enrolled, 2 were not living with their mothers and one was declined enrollment by the mother. Among the 160 pregnancies in DW, 140 (86.4%) babies were alive and enrolled, 5 (3.1%) were not enrolled, and for 17 (10.5%) pregnancies had adverse outcomes (9 post-natal deaths, 3 still births and 5 miscarriages). Among the 5 infants of DW who were not enrolled, 3 were not living with their mothers, 1 was declined enrollment and 1 baby was missing/kidnapped (Fig 2). None of the babies was found to be more than 9 moths (36 weeks) of age. There were no statistically significant differences in the occurrence of adverse pregnancy outcomes between RW and DW (P = 0.314).
Fig 2. Study consort diagram showing infants born to HIV positive retained women (RB) and disengaged women (DB) enrolled in a community outreach study between July 2017 –July 2018 from six Kampala City Council public municipal clinics in Uganda.
Infants born to retained and disengaged women were similar in gender distribution and age at enrollment with a median of 6 months and similar proportions in both groups were exclusively breast feeding at the time of study enrollment. A significantly lower proportion of babies of DW (38; 27.1%) were enrolled in the early infant diagnosis programs compared to 144 of the babies born to RW (96.6%; P<0.001), (Table 3). Among 138 of the babies of DW with an HIV test, 6 (4.3%) tested positive for HIV compared to 2 among 146 (1.4%) babies of RW (P = 0.162) (Table 3). Among 39 DW who delivered prior to disengagement from care one baby was found to be HIV-positive (MTCT rate 2.6%), compared to 5 babies (MTCT rate 5.1%) among women who delivered after disengagement.
Table 3. Demographic, clinical characteristics and outcomes of babies born to retained and disengaged women in a PMTCT program in Uganda.
| Characteristic | Babies of Retained Women (N = 149) | Babies of Disengaged Women (N = 140) | P Value |
|---|---|---|---|
| Age (months) at study enrollment, Median (IQR) | 6.0 (4.4, 7.5) | 5.4 (4.4, 6.7) | 0.282 |
| Male sex, n (%) | 84 (56.4) | 74 (52.9) | 0.548 |
| Weight in Kg at birth, Median (IQR) | 3.2 (3.0, 3.5) | 3.0 (2.9, 3.5) | 0.259 |
| Place of birth, n (%) | |||
| Health facility | 145 (97.3) | 130 (92.9) | 0.205 |
| Home | 3 (2.0) | 8 (5.7) | |
| Not Documented | 1 (0.7) | 2 (1.4) | |
| Infant feeding at study enrollment, n (%) | |||
| Exclusively breastfeeding | 68 (45.6) | 56 (40.0) | 0.334 |
| Enrolled in Early Infant Diagnosis care1, n (%) | 144 (97.9) | 38 (27.5) | <0.001 |
| Infant outcomes | |||
| Median weeks at PCR testing | 24 (19, 35) | 22 (21, 34) | 0.146 |
| HIV PCR Positive2, n (%) | 2 (1.4) | 6 (4.3) | 0.163 |
| HIV PCR positivity by timing of delivery, n (%) | N/A | N/A | |
| Delivered before mother disengaged | 1/39 (2.6) | ||
| Delivered after mother disengaged | 5/99 (5.1) |
1Out of 147 retained babies and 138 disengaged babies.
2Unusable samples for HIV PCR test for 3 retained and 2 disengaged babies.
Discussion
In our study, we observed that nearly half of women engaged in PMTCT programs had disengaged from care. These estimates were comparable to countrywide evaluation of the Option B+ program which reported similar loss-to-follow-up among pregnant women initiating ART in Uganda [29]. Despite the intensive outreach of disengaged pregnant women enrolled in the PMTCT program, we were only able to ascertain outcomes of 44.6% of these women. Tracing studies in non-pregnant adults have reported higher rates of successful tracing, several with over 80% outcome ascertainment for the outreached population [21,30,31]. In eSwatini, community tracing of women who were LTFU ascertained outcomes in 44.7% of women [23], while in Malawi only 40% of women LTFU in Option B+ programs were successfully traced [24]. These low rates of outcome ascertainment highlight the challenges of community tracing of WLWHIV in SSA.
One of the most common reasons for failure to ascertain outcomes of the pregnant women may be their high mobility. High mobility and migration of persons living in urban communities is attributed to the search for employment, particularly among young adults [32,33]. Among pregnant women, nearly half return to their original home for delivery and family support during the postpartum period [34]. This contributes to the difficultly in locating DW. In a study in South Africa, the majority of women traveled away from the city to the villages of origin for a relatively short time (median of 32 days), and planned to return to their original health facility in the city, while infant care was often delegated to the grandparents [34]. Understanding the mobility patterns of postpartum women thus remains critical for ensuring continuity of care for WLWHIV and their exposed infants. Additionally, it is important to identify and address resources and relational health system barriers to retention in care among WLWHIV [35,36].
As expected, we observed that a higher (61.0%) proportion of DW had detectable VL as compared to RW (10.4%). This was the case even among women who reported receiving care at another facility, of whom 28.4% had unsuppressed virus compared to 9.7% for matched RW. In a recent study in Zambia, viremia was present in 18.1% of people living with HIV/AIDS (PLWHV) who were retained in care, 71.3% in those LTFU, 49.8% of those lost and in care elsewhere and 83.9% of those lost and not in care [37]. Such high levels of unsuppressed viremia among DW increases the risk of HIV vertical transmission [38]. We postulate the rates of viral non-suppression for untraceable women are as high or higher than those who were successfully traced. This is assumption is based on the results from Sikazwe et al, where they found that 71.3% of persons who were lost had high viremia compared to those retained [34]. The low ascertainment of outcomes among WLWHIV who disengage from care underscores the need for strategies that identify women who are likely to disengage from care soon after ART initiation and that ensure retention, particularly during the postpartum period.
In our study, we found that 67.5% of the DW were out of care and had not received any ART since their last clinic encounter, while 32.5% had self-transferred to other health facilities. Our estimate of self-transfer was higher than that reported in a systematic review of data from 23 low-income and middle-income countries where 18.6% of the persons LTFU had silently transferred [39]. Our data cannot shed light as to whether this difference in our experience is a fact inherent to patterns of care seeking behavior in our setting or simply a positive bias in our estimates due to the large number of women whom we were unable to trace (if these women have lower probability of accessing care after dropping out from their original healthcare facility). Nevertheless, we found that 28.4% of the women who reported being in care elsewhere had unsuppressed viremia. PLWHIV who self-transfer face challenges of continuing ART at new health facilities and this is likely to affect adherence to treatment [34,37]. In fact, in our study, among women who reported that they had transferred, nearly a third had undetectable ART drug concentrations compared to 10% among women who were retained in care (P = 0.022). This discordance may represent issues related to women falsely reporting that they are in care based on social desirability. Studies have shown large discordances between ART detection and self-report adherence, and such discrepancies are more common among young adults and the results may be subject to social desirability bias [40,41]. Health care workers should emphasize the importance of continuity in care among WLWHIV. WLWHIV should be encouraged to seek ART services at facilities that are convenient to them in order to and avoid complete disengagement from care.
Although our study did not have adequate power to detect differences in vertical transmission of HIV, our findings suggest that higher transmission in infants born to DW as compared to RW. Our estimates of HIV transmission among DW were higher than that reported in a study conducted among women who disengaged from care in Zimbabwe, where the observed estimates of 3.6% were reduced to 1.8% following a tracing program which used community workers to ensure that all HIV positive pregnant women were in care throughout the post-partum period [22]. Among the women retained in care, our estimates of vertical transmission are slightly lower than Uganda national estimates of 2.9% [42]. In the Southern Africa, comparable rates of infant HIV infection (3%) were observed at 12 months and HIV-free survival was estimated at 97.5% at 6 months [43]. Even with the scarcity of prior studies reporting HIV vertical transmission among DW, our findings are likely optimistic as there are concerns that women that couldn’t be traced might have higher transmission rates for reasons described earlier. These results highlight the value of outreach activities for pregnant and breastfeeding WLWHIV.
Study strengths and limitations
One of the strengths of our study is that it contributes to our understanding of HIV outcomes among pregnant and breastfeeding women who disengage from HIV programs in SSA. Our findings are generalizable to populations in urban areas in SSA with great population mobility. We also acknowledge some limitations of our study. From the electronic health records, almost half of women who were identified as disengaged from care were not successfully contacted, leading to a much smaller sample size than was initially planned. Because of this, we were underpowered to fully explore questions related to MTCT risk factors and drug concentrations. Also, the differences in the months of postpartum led to different amounts of person time which could be have contributed to the non-statistical difference in MTCT. More importantly, some of our findings may be biased because of a strong association between an outcome (e.g. re-engagement in care) and whether a woman was successfully traced. In addition, our study cannot address outcomes in less mobile, non-urban settings.
Conclusions
High rates of disengagement from care were observed among women enrolled in PMTCT programs in Uganda. Pregnant and breastfeeding women who disengage from care are difficult to find in urban environments and a large proportion of the traceable women are out of care and have detectable viral loads leading to the potential for an increased risk of MTCT. In the current era of “Treat All”, development of strategies that enhance retention, mechanisms that track silent and unofficial transfers, as well as estimation of viral suppression among persons who disengage from care are critical. Customized interventions are needed to target pregnant women initiating ART that address several psychosocial and structural barriers to retention, particularly in the post-partum period. These results underscore the need for community tracing of women living with HIV following disengagement from care and highlight the poor outcomes associated with dropout for both mothers and their infants. Our study findings were used to inform a national campaign titled “Free to Shine Campaign” which which aimed to keep mothers and babies in care, and to prevent new HIV infections [44]. Similar campaigns coupled with tracing efforts will be critical for successful elimination of MTCT in resource-limited settings.
Supporting information
(CSV)
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
This work was supported by the National Institutes of Health (NIH) under the U01 AI069911 Eastern Africa International epidemiology Databases to Evaluate AIDS (IeDEA). Salary support for ANK was provided from the Fogarty International Center grant HIV co-infections (D43 TW009771) and the EDCTP (100448 TMA 2015 CDF - 1036.
References
- 1.WHO. Implementation of Option B+ for prevention of mother to child transmission of HIV: The Malawi experience. 2014.
- 2.UNAIDS. Start Free Stay AIDS Free: 2017 Progress Report. 2017.
- 3.UNAIDS. UNAIDS DATA 2018. 2018.
- 4.Haas AD, van Oosterhout JJ, Tenthani L, et al. HIV transmission and retention in care among HIV-exposed children enrolled in Malawi’s prevention of mother-to-child transmission programme. J Int AIDS Soc. September 4 2017;20(1):21947. 10.7448/IAS.20.1.21947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kiwanuka G, Kiwanuka N, Muneza F, et al. Retention of HIV infected pregnant and breastfeeding women on option B+ in Gomba District, Uganda: a retrospective cohort study. BMC Infect Dis. October 24 2018;18(1):533. 10.1186/s12879-018-3450-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Woelk GB, Ndatimana D, Behan S, et al. Retention of mothers and infants in the prevention of mother-to-child transmission of HIV programme is associated with individual and facility-level factors in Rwanda. J Int AIDS Soc. 2016;19(5 Suppl 4):20837. 10.7448/IAS.19.5.20837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Geldsetzer P, Yapa HM, Vaikath M, et al. A systematic review of interventions to improve postpartum retention of women in PMTCT and ART care. J Int AIDS Soc. 2016;19(1):20679. 10.7448/IAS.19.1.20679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.UNAIDS. Start Free Stay Free AIDS Free: 2017 progress report. 2017.
- 9.UNAIDS. Start Free Stay AIDS Free: 2020 Progress Report. 2020.
- 10.Kim MH, Ahmed S, Hosseinipour MC, et al. Implementation and operational research: the impact of option B+ on the antenatal PMTCT cascade in Lilongwe, Malawi. J Acquir Immune Defic Syndr. April 15 2015;68(5):e77–83. 10.1097/QAI.0000000000000517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tippett Barr BA, van Lettow M, van Oosterhout JJ, et al. National estimates and risk factors associated with early mother-to-child transmission of HIV after implementation of option B+: a cross-sectional analysis. Lancet HIV. Dec 2018;5(12):e688–e695. 10.1016/S2352-3018(18)30316-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sagay AS, Ebonyi AO, Meloni ST, et al. Mother-to-Child Transmission Outcomes of HIV-Exposed Infants Followed Up in Jos North-Central Nigeria. Curr HIV Res. 2015;13(3):193–200. 10.2174/1570162x1303150506182534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pricilla RA, Brown M, Wexler C, Maloba M, Gautney BJ, Finocchario-Kessler S. Progress Toward Eliminating Mother to Child Transmission of HIV in Kenya: Review of Treatment Guidelines Uptake and Pediatric Transmission Between 2013 and 2016-A Follow Up. Matern Child Health J. December 2018;22(12):1685–1692. 10.1007/s10995-018-2612-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Commission UA. Uganda HIV/AIDS Country Progress Report July 2016—June2017. 2017.
- 15.Kyaw KWY, Oo MM, Kyaw NTT, et al. Low mother-to-child HIV transmission rate but high loss-to-follow-up among mothers and babies in Mandalay, Myanmar; a cohort study. PLoS One. 2017;12(9):e0184426. 10.1371/journal.pone.0184426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nachega JB, Uthman OA, Anderson J, et al. Adherence to antiretroviral therapy during and after pregnancy in low-income, middle-income, and high-income countries: a systematic review and meta-analysis. AIDS. October 23 2012;26(16):2039–2052. 10.1097/QAD.0b013e328359590f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haas AD, Msukwa MT, Egger M, et al. Adherence to Antiretroviral Therapy During and After Pregnancy: Cohort Study on Women Receiving Care in Malawi’s Option B+ Program. Clin Infect Dis. November 1 2016;63(9):1227–1235. 10.1093/cid/ciw500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Geng EH, Bangsberg DR, Musinguzi N, et al. Understanding reasons for and outcomes of patients lost to follow-up in antiretroviral therapy programs in Africa through a sampling-based approach. J Acquir Immune Defic Syndr. 2010;53(3):405–411. 10.1097/QAI.0b013e3181b843f0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Geng EH, Emenyonu N, Bwana MB, Glidden DV, Martin JN. Sampling-based approach to determining outcomes of patients lost to follow-up in antiretroviral therapy scale-up programs in Africa. JAMA. August 6 2008;300(5):506–507. 10.1001/jama.300.5.506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Geng EH, Glidden DV, Bangsberg DR, et al. A causal framework for understanding the effect of losses to follow-up on epidemiologic analyses in clinic-based cohorts: the case of HIV-infected patients on antiretroviral therapy in Africa. American journal of epidemiology. May 15 2012;175(10):1080–1087. 10.1093/aje/kwr444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Geng EH, Odeny TA, Lyamuya R, et al. Retention in Care and Patient-Reported Reasons for Undocumented Transfer or Stopping Care Among HIV-Infected Patients on Antiretroviral Therapy in Eastern Africa: Application of a Sampling-Based Approach. Clin Infect Dis. April 1 2016;62(7):935–944. 10.1093/cid/civ1004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vogt F, Ferreyra C, Bernasconi A, et al. Tracing defaulters in HIV prevention of mother-to-child transmission programmes through community health workers: results from a rural setting in Zimbabwe. J Int AIDS Soc. 2015;18:20022. 10.7448/IAS.18.1.20022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reidy W, Nuwagaba-Biribonwoha H, Shongwe S, et al. Engagement in care among women and their infants lost to follow-up under Option B+ in eSwatini. PloS one. 2019;14(10):e0222959. 10.1371/journal.pone.0222959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tweya H, Gugsa S, Hosseinipour M, et al. Understanding factors, outcomes and reasons for loss to follow-up among women in Option B+ PMTCT programme in Lilongwe, Malawi. Trop Med Int Health. November 2014;19(11):1360–1366. 10.1111/tmi.12369 [DOI] [PubMed] [Google Scholar]
- 25.MOH. The Integrated National Guidelines on Antiretroviral Therapy, Prevention of Mother to Child Transmission of HIV and Infant & Young Child Feeding, 2012. 2012.
- 26.MOH U. National antiretroviral treatment and care guidelines for adults and children. 2008.
- 27.Phillips TK, Orrell C, Brittain K, Zerbe A, Abrams EJ, Myer L. Measuring retention in HIV care: the impact of data sources and definitions using routine data. Aids. April 1 2020;34(5):749–759. 10.1097/QAD.0000000000002478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.http://emrportal.mets.or.ug. UGANDA EMR. 2019. Accessed 6th May, 2019.
- 29.Muhumuza S, Akello E, Kyomugisha-Nuwagaba C, et al. Retention in care among HIV-infected pregnant and breastfeeding women on lifelong antiretroviral therapy in Uganda: A retrospective cohort study. PloS one. 2017;12(12):e0187605. 10.1371/journal.pone.0187605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Geng EH, Glidden DV, Bwana MB, et al. Retention in care and connection to care among HIV-infected patients on antiretroviral therapy in Africa: estimation via a sampling-based approach. PLoS One. 2011;6(7):e21797. 10.1371/journal.pone.0021797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Geng EH, Odeny TA, Lyamuya RE, et al. Estimation of mortality among HIV-infected people on antiretroviral treatment in East Africa: a sampling based approach in an observational, multisite, cohort study. Lancet HIV. March 2015;2(3):e107–116. 10.1016/S2352-3018(15)00002-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Anglewicz P. Migration, marital change, and HIV infection in Malawi. Demography. February 2012;49(1):239–265. 10.1007/s13524-011-0072-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schuyler AC, Edelstein ZR, Mathur S, et al. Mobility among youth in Rakai, Uganda: Trends, characteristics, and associations with behavioural risk factors for HIV. Glob Public Health. August 2017;12(8):1033–1050. 10.1080/17441692.2015.1074715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Clouse K, Fox MP, Mongwenyana C, et al. "I will leave the baby with my mother": Long-distance travel and follow-up care among HIV-positive pregnant and postpartum women in South Africa. Journal of the International AIDS Society. July 2018;21 Suppl 4:e25121. 10.1002/jia2.25121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oral abstracts of the 10th IAS Conference on HIV Science, 21–24 July 2019, Mexico City, Mexico. Journal of the International AIDS Society. July 2019;22 Suppl 5:e25327. 10.1002/jia2.25327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marotta C, Giaquinto C, Di Gennaro F, et al. Pathways of care for HIV infected children in Beira, Mozambique: pre-post intervention study to assess impact of task shifting. BMC public health. June 7 2018;18(1):703. 10.1186/s12889-018-5646-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sikazwe I, Eshun-Wilson I, Sikombe K, et al. Retention and viral suppression in a cohort of HIV patients on antiretroviral therapy in Zambia: Regionally representative estimates using a multistage-sampling-based approach. PLoS Med. May 2019;16(5):e1002811. 10.1371/journal.pmed.1002811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Landes M, van Lettow M, Nkhoma E, et al. Low detectable postpartum viral load is associated with HIV transmission in Malawi’s prevention of mother-to-child transmission programme. Journal of the International AIDS Society. June 2019;22(6):e25290. 10.1002/jia2.25290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wilkinson LS, Skordis-Worrall J, Ajose O, Ford N. Self-transfer and mortality amongst adults lost to follow-up in ART programmes in low- and middle-income countries: systematic review and meta-analysis. Trop Med Int Health. March 2015;20(3):365–379. 10.1111/tmi.12434 [DOI] [PubMed] [Google Scholar]
- 40.Mooney AC, Campbell CK, Ratlhagana MJ, et al. Beyond Social Desirability Bias: Investigating Inconsistencies in Self-Reported HIV Testing and Treatment Behaviors Among HIV-Positive Adults in North West Province, South Africa. AIDS Behav. July 2018;22(7):2368–2379. 10.1007/s10461-018-2155-9 [DOI] [PubMed] [Google Scholar]
- 41.Huerga H, Shiferie F, Grebe E, et al. A comparison of self-report and antiretroviral detection to inform estimates of antiretroviral therapy coverage, viral load suppression and HIV incidence in Kwazulu-Natal, South Africa. BMC infectious diseases. September 29 2017;17(1):653. 10.1186/s12879-017-2740-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.UNAIDS. 2015 Progress report on the Global Plan towards elimination of new HIV infections among children and keeping their mothers alive. 2015.
- 43.Chi BH, Mutale W, Winston J, et al. Infant Human Immunodeficiency Virus-free Survival in the Era of Universal Antiretroviral Therapy for Pregnant and Breastfeeding Women: A Community-based Cohort Study From Rural Zambia. Pediatr Infect Dis J. November 2018;37(11):1137–1141. 10.1097/INF.0000000000001997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.https://observer.ug/images2/Ads/Docs/Free-to-Shine-Campaign.pdf. The Observer2018.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(CSV)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.