The ability of ecosystems to withstand disturbances and maintain their functions is being increasingly tested as rates of change intensify due to climate change and other human activities. Microorganisms are crucial players underpinning ecosystem functions, and the recovery of microbial communities from disturbances is therefore a key part of the complex processes determining the fate of ecosystem functioning.
KEYWORDS: aquatic, compounded, disturbance, microbial communities, resilience, soil, terrestrial
SUMMARY
The ability of ecosystems to withstand disturbances and maintain their functions is being increasingly tested as rates of change intensify due to climate change and other human activities. Microorganisms are crucial players underpinning ecosystem functions, and the recovery of microbial communities from disturbances is therefore a key part of the complex processes determining the fate of ecosystem functioning. However, despite global environmental change consisting of numerous pressures, it is unclear and controversial how multiple disturbances affect microbial community stability and what consequences this has for ecosystem functions. This is particularly the case for those multiple or compounded disturbances that occur more frequently than the normal recovery time. The aim of this review is to provide an overview of the mechanisms that can govern the responses of microbes to multiple disturbances across aquatic and terrestrial ecosystems. We first summarize and discuss properties and mechanisms that influence resilience in aquatic and soil biomes to determine whether there are generally applicable principles. Following, we focus on interactions resulting from inherent characteristics of compounded disturbances, such as the nature of the disturbance, timing, and chronology that can lead to complex and nonadditive effects that are modulating the response of microorganisms.
INTRODUCTION
The ability of aquatic and terrestrial ecosystems to withstand disturbances and maintain ecosystem functions is being increasingly tested as rates of environmental change intensify due to climate change and other human activities. The drivers of change are diverse and may differ from ecosystem to ecosystem. For example, 20% of China’s farmland is contaminated with heavy metals, and salinity is reducing the production potential of up to 46 M ha yr−1 (1). In Europe, there are more than 650,000 terrestrial sites that are expected to be contaminated (2), while assessment of 1,511 sites in Europe’s seas revealed that 93% of them are contaminated by hazardous substances (3). Although individual trajectories may differ, air, water, and soil pollution have continued to increase, and the compounding effects of drivers such as climate change, land-/sea-use change, overexploitation of resources, and pollution are likely to exacerbate the negative impacts on ecosystem functions (4). Therefore, there is a growing interest in understanding the cumulative impact of compounded disturbances in the world’s ecosystems, since interactions between multiple disturbances can generate complex and nonadditive effects (5, 6).
Microorganisms are crucial players underpinning ecosystem functions, primarily because of their involvement in biogeochemical cycling, in marine (7), freshwater (8), and terrestrial (9) ecosystems. While it has been stated that microbial diversity is never so impoverished that the microbial community cannot play a full part in biogeochemical cycling (8), more recent studies have linked loss of microbial taxa to the impairment of ecosystem functions (10, 11). Furthermore, the importance of biological diversity for ecosystem functioning increases as more functions, i.e., multifunctionality, are considered, a trend that is consistent throughout aquatic and terrestrial ecosystems (12–14). The recovery of microbial communities from disturbance is, therefore, a key part of the complex processes determining the fate of ecosystem functioning in response to disturbances resulting from changing climatic conditions and increasing human activities (15). However, despite global environmental change consisting of numerous pressures, most studies have investigated only one or two factors, and the effects of compounded disturbances on microbial community composition and functions are unclear and controversial (5, 6) (Fig. 1). Previous reviews of microbial resilience and stability focused mostly on the effects of a single disturbance to provide excellent insight into the drivers of microbial community stability (15–17). Here, we therefore focus instead on compounded disturbances and aim to provide an overview of the mechanisms that can govern the responses of microbes to multiple drivers of global change and how interactions between disturbances can modulate the response to disturbance. We first summarize contributions from recent studies related to microbial resilience mechanisms and discuss ecosystem-specific properties and mechanisms that influence resilience in aquatic (more specifically, pelagic) and soil ecosystems to determine whether there are generally applicable principles. Based on this, we then discuss the effects of multiple disturbances and highlight the importance of the inherent characteristics of sequences of disturbances in determining the disturbance responses of microbial communities.
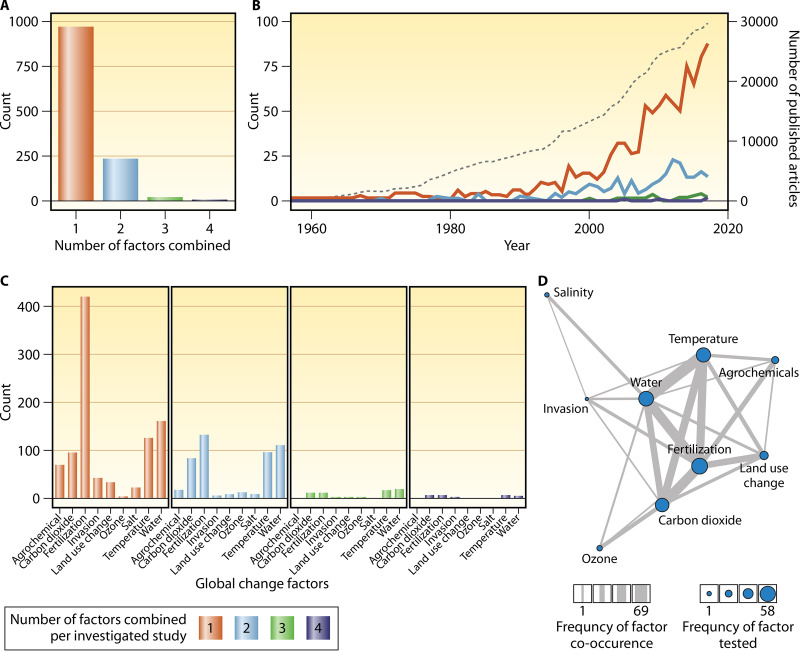
FIG 1.
(A) Frequency distribution of the number of factors of global change included in experimental studies between 1957 and 2017. (B) Numbers of experimental studies that included a given number of factors over the past 50 years. For comparison, the dashed gray line (right y axis) represents the number of published articles per year for the Web of Knowledge category “ecology.” (C) Numbers of papers that included a given global change factor for studies with one to four combined factors. (D) Network graph depicting the co-occurrence of global change factors in experimental studies, where circle size represents the frequency with which the driver was included in the studies, and line thickness represents the frequency with which the drivers were tested as combinations. Reproduced from reference 6 with permission from AAAS.
RESILIENCE, A FUZZY CONCEPT
The whole resilience subject area is fraught with myriad definitions and the broadened understanding through general use of originally tightly defined terms (such as “resilience” itself) (18). Resilience to a disturbance can be defined in two ways: (A) as engineering resilience, which is the rate at which a system returns to the original state, and (B) as ecological resilience, which is the disturbance required to move the system from one stable to another, i.e., alternative, stable state (17). Most studies in microbial ecology have so far used the engineering resilience concept (17). Generally, it has been suggested that the terms “resistance and recovery” should be used to assess resilience (19, 20), and there is also increasing recognition that the two concepts (ecological and engineering resilience) have many synergies when different temporal and spatial scales are considered (21) and should be unified (22). Song et al. (23), for example, sought to reconcile the engineering and ecological definitions of resilience in microbial communities by recognizing that they display both elastic (i.e., engineering) and plastic (i.e., ecological) resilience and advocated combining the two concepts by a focus on functional resilience. Likewise, Todman et al. (24) took a more general approach and defined four quantitative resilience metrics (degree of return, return time, rate of return, and efficiency) that together with resistance can be used to describe the response and recovery of microbial communities to disturbance. This is also in line with the recognition that stability is a multidimensional concept (25) that needs to include several descriptors, such as resistance, recovery, (engineering) resilience, and temporal stability, the latter reflecting that recovery is not necessarily a smooth trajectory but can vary in time (26) (Fig. 2). It is also important to highlight that accurate differentiation and quantification of resilience and stability metrics require the collection of highly resolved time series data after a disturbance.
FIG 2.
Conceptual overview of compositional and functional responses of microbial communities to a disturbance. Initially, both microbial community composition and function change in response to the disturbance, where resistance refers to the degree of initial change. Subsequently, four simplified scenarios for recovery are possible: A, complete recovery; B, only composition recovers but not function (physiological adaptation); C, only function recovers but not composition (functional redundancy); and D, no recovery. Resistance, recovery, recovery rate (engineering resilience), and temporal stability are 4 aspects that describe the overall compositional and functional resilience or stability of the community (25, 26) and are expected to be influenced by disturbance, community, and habitat properties that, in addition, also modulate effects of community assembly processes on resilience. In this review, the term microbial resilience is used in the broadest sense to encompass the vast variety of definitions used in the literature (see the text) and mainly covers resistance, recovery, and engineering resilience, as the majority of studies in microbial ecology have focused on these metrics.
Since microbial communities can be resilient in terms of their composition, functioning, or both (Fig. 2), 4 extreme scenarios have been used to describe the compositional and functional recovery from a disturbance (27): full recovery (both composition and function recover to the original state), full physiological adaptation (composition recovers but function does not), full functional redundancy (function recovers but composition does not), and no recovery (neither composition nor function recover to the original state). More realistically, trajectories of incomplete recovery are likely to be found due to, for example, shifts in baseline environmental conditions or ecosystem succession that prevent return to the original state (28). Moreover, different mechanisms that underlie resilience, such as physiological plasticity, dispersal, and evolutionary adaptation, operate at different time scales (Fig. 3), which can influence the resilience of different groups of microorganisms with different life spans and also cause time lags between functional and compositional responses to disturbance.
FIG 3.
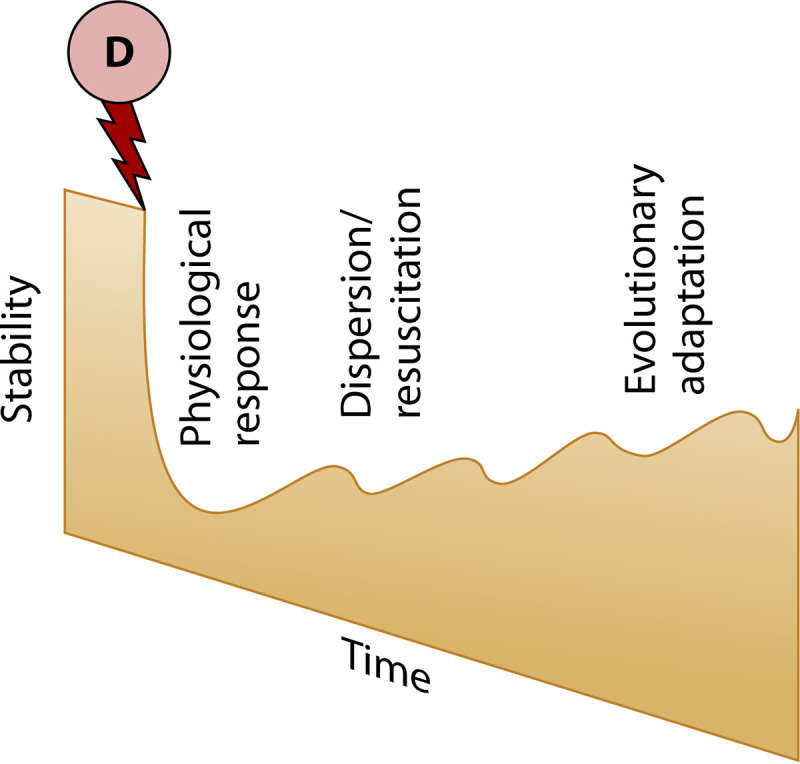
Schematic illustration of how the underlying mechanisms contributing to microbial resilience (stability) operate at different time scales.
In summary, as resilience remains a fuzzy concept, our recommendation would be to clearly define in each study what is meant by terms related to resilience. In this review we use the term resilience in the broadest sense in alignment with the recent developments to integrate engineering and ecological resilience and recognition that resilience and stability are multidimensional concepts. Hence, we include multiple characteristics that describe both the initial displacement and the return to the original state (here referred to simply as “resilience”) and try to be as explicit as possible about the specific descriptors we refer to and/or were analyzed in the examples provided (see Table 1). The chosen examples are primarily based on resistance, recovery, and engineering resilience, as the majority of studies in microbial ecology have used these descriptors.
TABLE 1.
Glossary terms
| Term | Definition | Reference(s) |
|---|---|---|
| Bayesian | Based on Bayes’ theorem that describes the probability of an event based on prior knowledge of conditions that might be related to the event | 152 |
| Community assembly | ||
| Coalescence | An emerging paradigm unique to microorganisms that considers the wholesale mixing of multiple communities and their surrounding environments, which can occur on a regular or intermittent basis and within a short period of time | 96 |
| Community | Group of organisms representing multiple species living in a specified place and time | 29 |
| Dispersal | The movement of organisms across space | 29 |
| Diversification | Changing phylogenetic diversity resulting from genetic and environmental variabilities | 153 |
| Drift | Random changes in the relative abundance of species due to chance variation amongst individuals | 29 |
| Priority effect | The order and timing of species immigration during community assembly can affect community composition | 154 |
| Selection | A deterministic fitness difference between individuals of different species in response to local abiotic and biotic conditions | 29 |
| Speciation | The creation of new species | 29 |
| Disturbance | A discrete unpredictable event that causes direct removal of living biomass, thereby altering community structure | 155 |
| Compounded disturbance | Multiple disturbances, either simultaneously or more frequent than the normal recovery time | 111 |
| Press disturbance | Transient, acute; relatively long-term, continuous event | 16, 117 |
| Pulse disturbance | Persistent, chronic; relatively discrete, short-term event | 16, 117 |
| Perturbation | Change in the level of function or population of a system due to a disturbance | 24 |
| Ecological network | Representation of possible links between species (nodes) within an ecosystem | 73 |
| Network connectance | Proportion of possible links between species (nodes) that are realized | 156 |
| Network modularity | The tendency of a network to be compartmented into separated clusters of interacting nodes | 157 |
| Niche filtering | A concept whereby the environment functions like a filter (or sieve) removing all species lacking specified combinations of traits | 158 |
| Node centrality | A measure of the importance of the node within the network | 73 |
| Ecosystem function | The biological, geochemical, and physical processes that take place or occur within an ecosystem | 159 |
| Functional redundancy | Where multiple species representing a variety of taxonomic groups can share similar roles in providing ecosystem functions | 160 |
| Multifunctionality | The ability of an ecosystem to provide multiple functions | 161 |
| Mycorrhizal fungi | Fungi forming a symbiotic association with plants | 162 |
| Resilience | ||
| Ecological resilience | Measure of disturbance required to move system from one stable state to another | 17 |
| Engineering resilience | Rate of recovery towards postdisturbance state | 17 |
| Resistance | Initial ability of a system to withstand disturbance | 17 |
| Stability | Inherent property of a system to remain unchanged in response to disturbance. Combines resistance, recovery, (engineering) resilience, and temporal stability | 17, 25, 26 |
| Temporal stability | The inverse of the variability around functional and compositional trajectories during the recovery phase | 26 |
| Rhizodeposition | The release of organic compounds from plant roots into the surrounding environment | 163 |
| Succession | ||
| Autogenic | Ecological succession driven by biotic factors | 164 |
| Secondary | Succession that follows a disturbance | 165 |
WHAT MATTERS FOR MICROBIAL RESILIENCE?
Compositional resilience is influenced by properties of individual cells, populations, and communities (16), where lower level properties define aggregated properties at higher levels. Similarly, functional resilience is thought to be governed by properties that operate across three scales: the species level (e.g., sensitivity, growth rate, versatile physiology, and genetic variability), the community level (e.g., trait composition, functional redundancy, and network structure), and the landscape level (e.g., environmental heterogeneity and connectivity/dispersal) (16). Here, we focus on processes at the community and landscape levels that are relevant for the resilience of microbial communities in aquatic and terrestrial ecosystems with the overall aim to depict similarities as well as potential differences in the underlying mechanisms between these environments.
Microbial Community Assembly Processes
Patterns in ecological communities arise as a result of four processes: speciation, selection, dispersal, and drift (29). The unique combinations of these assembly processes influence not only the composition of microbial communities in time and space but also, in interaction with intrinsic community properties and traits (see below), their response to disturbances. Ecological drift is the stochastic change in the relative abundance of species due to chance variation among individuals (29). However, this process is difficult to quantify for microbial communities due to methodological issues and is often confused with apparently stochastic changes in taxonomic composition and interwoven with speciation and dispersal. Therefore, it will not be discussed further.
Diversification/speciation.
Short generation time, high frequency of homologous recombination, gene exchange, gene loss, and mutations altogether with large population sizes are all key ingredients supporting diversification as a driver of microbial community composition following disturbance. All are, therefore, of importance for the compositional resilience of microbial communities. Diversification can be driven by the selection pressure of the disturbance itself, resulting in a process known as adaptive radiation in evolutionary biology. For example, the diversification of the ubiquitous phylum Thaumarchaeota was suggested to be coupled to pH adaptation in soils (30). Furthermore, diversification in the model bacterium Pseudomonas aeruginosa increased the functional resistance of biofilms to an oxidative stress compared to that of biofilms formed by mutants that were unable to diversify (31). This example supports the so-called “insurance hypothesis,” which predicts that communities that are more diverse are more likely to contain species that can cope with a disturbance and maintain functioning even if others fail (32). On the other hand, there are also examples where disturbance hinders diversification. For example, while increasing temperature promoted adaptive diversification in Pseudomonas fluorescens populations, low temperatures did not (33). Hence, it seems likely that the role of diversification for microbial resilience depends on the disturbance type.
Selection.
Due to the wide and versatile metabolic diversity among microbial taxa, it is evident that selection is an important shaping force of microbial communities that will favor microorganisms able to withstand or adapt to a disturbance. The importance of selection in the functional response of microbial communities to disturbance is also intrinsically linked to the insurance hypothesis as explained above, so that taxa that are resistant to the disturbance increase in abundance and compensate for functions previously carried out by sensitive taxa. In the case of microbes, selection can apply not only to individual cells but also to genetic mobile elements, so that the horizontal transfer of genes directly or indirectly contributes to resilience by accelerating adaptation of microbial communities to a disturbance (34). Particularly, metal resistance genes are often colocalized on plasmids within transposable and integrative mobile elements together with antibiotic resistance genes (35), and comparative genomic studies of Rhodanobacter strains from heavy-metal-contaminated groundwater showed evidence of lateral gene transfer and/or duplication of several metal resistance genes (36).
Dispersal.
Microorganisms are well known for being capable of long-distance dispersal by wind blow, water flows, or hitchhiking on other mobile organisms (37), and dispersed microorganisms represent an important regional species pool that can colonize disturbed local ecosystems. Successful colonization can be facilitated if disturbances open up niches previously occupied by nonresistant microorganisms and thereby play an important role for functional resilience. In addition, dispersal can also enhance compositional recovery by reintroducing taxa that were lost after a pulse disturbance. For example, Székely and Langenheder (38) experimentally demonstrated that dispersal at an early stage of rewetting promoted recovery of water bodies by reintroducing bacterial taxa that were lost after drought episodes. Similarly, the resilience of bacterial communities in soil mesocosms exposed to thermal disturbances was shown to be enhanced by dispersal (39). However, it is difficult to distinguish the populations recovered through dispersal from those recovered by resuscitation of dormant organisms, and Sorensen and Shade (39) showed that both processes contribute to microbial resilience. Moreover, the effect of dispersal compensating for diversity losses may only be evident in communities of relatively low diversity (40), as the level of diversity of the native community has been shown to act as a barrier when there is competition for the same limiting resources (41). Therefore, it can intuitively be hypothesized that the importance of dispersal for microbial resilience is positively related to the biocidal impact of the disturbance (40, 42). Finally, the arrival order of immigrant species is also likely to be of importance for resilience due to priority effects, where species arriving first after a disturbance possibly drive successional trajectories of communities by hindering or facilitating the establishment of late-arriving species (43, 44).
Microbial Community Composition
There are several community properties and traits that can influence microbial resilience (16), and here, we summarize the effects of rarity, dormancy, growth strategies, and the taxonomic affiliations of community members (taxonomic composition) before we discuss biotic interactions.
Rarity.
Microbial communities typically consist of many rare and few abundant taxa at any given point in time but are highly dynamic, so that even rarer subordinate taxa can become dominant when conditions turn out to be more favorable for them (45). As such, it has been proposed that disturbances would allow rare species to increase in abundance (16), which can therefore maintain ecosystem functioning assuming that rare species support important functions. Recently, Kurm et al. (46) tested experimentally whether rare taxa were more likely to increase in abundance than dominant taxa in response to various disturbances (e.g., copper addition, heat shock, drying-rewetting, and freeze-thawing). They found that only 1% of the rare taxa but 12% of the dominant taxa increased in abundance after disturbance, which does not support the idea that rare taxa are predominant drivers of microbial resilience.
Dormancy.
The majority of fungi and several bacterial groups have the ability to enter dormant forms (e.g., cysts and spores); therefore, dormant microorganisms may play key roles in the recovery of microbial communities following disturbance by serving as “seed banks.” It has, for example, been shown that resuscitation of dormant taxa was key for community transition during a thermal disturbance and that both resuscitation of opportunistic taxa and immigration contributed to the recovery during secondary succession after the disturbances (39). Dormancy was also the main mechanism identified to be responsible for the resilience of eukaryotic microbial communities to drought in shallow freshwater ecosystems (47). The proportion of microorganisms capable to enter dormant forms after the first disturbance and thus are resistant to a second disturbance can therefore also modulate the response of microbial communities to multiple disturbances.
Growth strategies.
Taxa can be assigned as r and K strategists, where the former have faster growth and higher turnover rates than the latter (48). Due to their high growth rates, r strategists have the potential to recover quickly after disturbance and therefore contribute to resilience (49, 50). Accordingly, the relative abundance of r and K strategists was shown to partly explain the response of microbial community composition to climate change-related disturbances (51). For bacteria, it has been shown in pure cultures that the number of rRNA operons in bacteria is positively related to growth rates, and analyzing patterns in community-weighted mean 16S rRNA gene copies has therefore been applied as a method to directly quantify changes in growth strategies during postdisturbance succession (44, 52, 53). For example, in fire-affected soils, Kearns and Shade (53) not only showed that operon counts decreased at/during disturbance and then increased during recovery but also highlighted that operon counts need to be interpreted carefully, since there is limited information about how the growth strategies of most taxa relate to ribosomal operon counts in the environment.
Taxonomic composition.
Differences in the taxonomic affiliation of community members can also influence microbial resilience, as they determine the cumulative number and identity of traits present in a community. Accordingly, the literature provides examples showing that different taxonomic groups of microorganisms (e.g., soil microbes and fauna) respond differently to the same disturbance (54, 55). In terrestrial ecosystems, several studies compared the resilience of fungi and bacteria. For example, fungal richness and evenness increased during drought and rapidly returned to normal levels after rewetting, whereas bacterial richness and evenness decreased and did not recover during the study period, which covered 2 months after the drought period (56). Moreover, networks of bacterial communities (see below) were more strongly affected than fungal networks. In contrast, Meisner at al. (57) showed that experimental drying and extreme rewetting events affected only 8% of prokaryotic, i.e., bacterial and archaeal, operational taxonomic units (OTUs) but up to 25% of fungal OTUs. The mechanisms behind these different responses remain unclear but might, for example, be influenced by differences in growth rates between bacteria and fungi in response to specific disturbance regimes or be related to differences in their life spans.
To which extent microbial community composition is important for ecosystem functioning in response to a disturbance is still debated (58), in particular, in relation to the concept of functional redundancy. Empirical evidence shows that higher phylogenetic dissimilarities among species within a community increases the probability of finding more species that can resist or recover from the disturbance, which can maintain ecosystem functioning. For example, using temperature and salt gradients as disturbances, Hallin et al. (59) demonstrated that the functional operating range was broader and denitrification rates faster in soil bacterial communities with the largest phylogenetic dissimilarity. Other studies have shown that differences in microbial community composition can explain differences in functional resilience between locations, suggesting a tight link between community composition and functional resilience. For example, community composition was the only variable among a range of biotic and abiotic environmental variables that accounted for functional resistance and engineering resilience to drought across a land-use gradient (60), although it was not important under undisturbed conditions. Similarly, resistance of multiple ecosystem functions linked to C, N, and P cycling to dry-wet cycles was regulated by microbial community composition across 59 dryland ecosystems (61).
Taking into account the temporal scale of microbial responses to disturbances might at least partly help to reconcile apparent discrepancies among studies investigating the impact of disturbances on community composition and functioning. For example, a mild disturbance is likely to initially affect microbial functions but not microbial community composition, whereas over a longer term, changes in community composition and deeper changes in functioning are likely to be found, as the underlying mechanisms operate at different time scales (Fig. 3). Given that growth and turnover rates in most environments are in the range of days (62), a faster functional than compositional response is somewhat expected and was, for example, illustrated in a study that compared functional and compositional responses of microbial communities from a freshwater rock pool to salinity disturbance (63). However, a delayed compositional response is also often interwoven with methodological limitations, as the DNA-based molecular approaches used in most studies have the limitation that they include relic DNA, i.e., extracellular DNA of dead cells, which can account for approximately 40% of both prokaryotic and fungal DNA (64). This relic DNA can obscure disturbance effects, temporal patterns, and therefore relationships between microbial composition and functioning. Hence, there is clearly a need for more studies that use approaches that specifically target active microorganisms (e.g., see reference 39).
Biotic Interactions
Biotic interactions and disturbances.
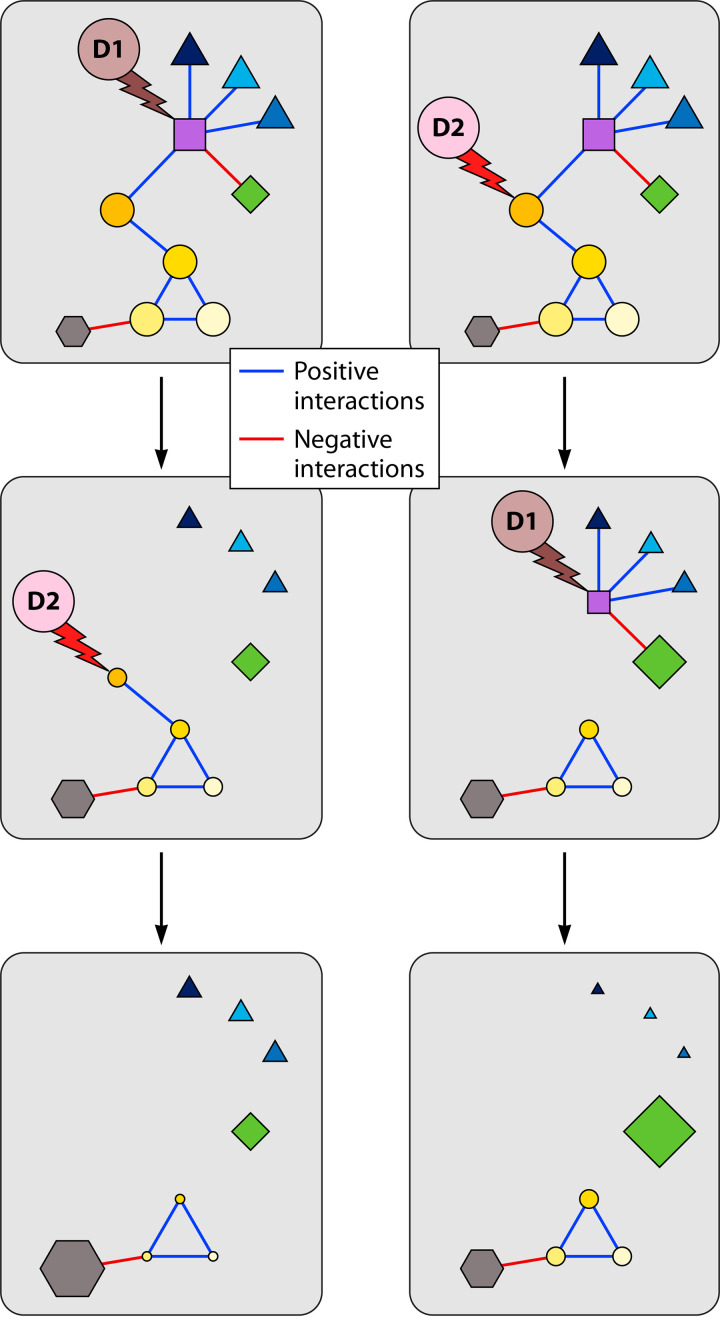
Microorganisms are known to interact with each other, which includes negative interactions, such as competition for space and resources, and positive interactions, such as the secretion of compounds that can promote the growth and survival of other cells around them (65–68). Therefore, a given disturbance will not only directly affect the fitness of susceptible taxa (i.e., those not resistant to the disturbance) but also indirectly affect the fitness of others that can resist the disturbance but interact with the susceptible ones (Fig. 4). This can lead to cascading effects and might even trigger secondary extinction (69). It can also explain some counterintuitive effects of disturbance leading to an increase in the abundance of some species as a consequence of the loss of a dominant competitor that could not withstand the disturbance (Fig. 4). In the case of multiple disturbances, the resulting communities might therefore be different depending on the connectedness of the affected species. Most disturbance studies do not distinguish such direct and indirect effects on microbes and only assess overall changes in community structure. As a consequence, both susceptible microorganisms and resistant microorganisms interacting with the susceptible ones are likely to be lumped together when long-term changes in community composition after disturbance are identified. One way to distinguish direct and indirect effects would be to monitor successional patterns at high temporal resolution after pulse disturbances, assuming that susceptible microorganisms respond first to the disturbance followed by those interacting with the susceptible taxa.
FIG 4.
Conceptual representation showing how two different disturbances (D1 and D2)—each impacting a different species—can alter microbial community composition when applied alone or sequentially combined. Symbols with shapes and colors represent different species. Under these two scenarios of disturbance chronology (left and right), the abundances of the species interacting directly or indirectly with the species lost after the disturbances are increasing or decreasing (represented by the size of the symbols) depending on the type of interactions.
Co-occurrence networks.
Microbial network analysis is increasingly used in microbial ecology to investigate associations between microorganisms within and across domains and is also employed to study the responses of microbes to disturbance (70). Hence, any change in network properties after disturbance might be due to changes in biotic interactions and/or selection effects associated with the disturbance if indirect links are not accounted for. Unfortunately, most often, putative interaction relationships in the inferred microbial association network lack further experimental evaluations. Network properties include several metrics, such as modularity, connectedness, and node centrality, that can have implications for microbial resilience. Seminal theoretical work in ecology predicted that more complex networks with a high number of interconnections are more likely to resist a disturbance. This view emerged from the early work of MacArthur (71) who concluded that stability increases as the number of species and interactions increases. Conversely, mathematical models demonstrated that network stability can decrease with diversity and complexity (71, 72). Furthermore, more recent theoretical work showed that higher connectedness decreased the modularity (which quantifies to what extent networks can be broken up into smaller components) and the engineering resilience of both trophic and mutualistic networks (73). While several theoretical articles have discussed the use of networks to assess the impact of a disturbance (74, 75), there are to date only few robust empirical studies that have linked network properties to microbial resilience. One example is a study that investigated the impact of drought in grassland mesocosms and revealed longer-lasting and stronger effects on bacterial networks than on fungal networks (56). More specifically, connectedness and node centrality increased in bacterial networks in response to drought while modularity decreased, suggesting a lower stability of bacterial networks in response to a drought disturbance. In shallow freshwater ecosystems, warming was found to significantly increase network size and nestedness of planktonic and benthic communities (76). Furthermore, the benthic nodes appeared to be capable of coping with the disturbances (highest resistance) and had the lowest recovery, which could be related to differences in the scale of ecological processes occurring in this habitat compared to those in the planktonic community.
Differences between Microbial Habitats
There are differences in habitat properties that are potentially of importance for microbial resilience because they influence disturbance impacts as well as community properties and assembly processes. In particular, habitat-specific differences in spatial and temporal heterogeneity and trophic structure could result in different resilience mechanisms. Here, we focus specifically on soil and pelagic (open water) systems as they are expected to differ most clearly in terms of the above-mentioned habitat properties. In this section, we therefore deliberately exclude microbial communities in sediments and biofilms in aquatic ecosystems from this comparison, as they might, in many ways, share more properties with soils than with pelagic systems.
Spatial and temporal heterogeneity.
One obvious difference between soil and pelagic environments is that spatial complexity is much higher in the former due to the presence of soil particles of different sizes (77). Hence, soil microorganisms experience a spatially structured environment with a heterogeneous distribution of resources and inhabit water-filled channels in pockets created by the spatial structure of soil particles, roots, fungal hypha, and animal excavations (78). While pelagic microorganisms experience a comparatively homogenous environment and are dominated by free-living cells (79), most soil microorganisms live in close contact with surfaces where they form aggregates of cells and soil particles rather than being freely suspended in the soil water (80). It has also been suggested that such aggregates serve as “evolutionary incubators” that form spatially isolated units that separate microbial cells from influences of the surrounding soil matrix, including a barrier against effects of certain disturbances and dispersal (67). The greater spatial complexity and resulting large number of microenvironments has been suggested as the main reason behind the higher diversity in soils than in pelagic systems (68, 81), and the nutrient-depleted and stressful conditions that can arise within aggregates might also be one reason why dormancy is more common in soils than in pelagic systems (82). Aggregates might provide a shelter for microbial communities against outside disturbances because they create an effective diffusion barrier that reduces exposure to, for example, pollutants, or because cells in the center of aggregates are protected against predation (67). Even in pelagic systems, aggregates can be important, as it has been shown that particles serve as refuges from which bacteria can recolonize the water column after pulse disturbances (83). Furthermore, it has been shown that a spatially structured environment can increase population resistance to antibiotic pulses because of increasing phenotypic variation and metabolic interactions among subpopulations (84), and similar mechanisms could explain why the destruction of soil structure by shaking reduced both resistance and engineering resilience of microbial communities (85).
The spatial structure of soils also results in spatially fragmented disturbances compared to those in pelagic systems. This applies, in particular, to disturbances such as drought, fire-induced temperature increases, contamination, and salt intrusion because their effects depend on pore connectivity as well as soil depth. Modeling studies have shown that a high spatial fragmentation of a disturbance can increase both compositional and functional resilience (86–90) under a range of different conditions (e.g., disturbance frequencies and intensities), possibly resulting from increasing “edge effects,” where a closer distance between disturbed and undisturbed areas leads to more rapid recolonization of disturbed areas and a faster recovery of populations (91). This also shows that dispersal is a key process for the recovery of biodiversity from spatially structured disturbances and that soil conditions resulting in a more clustered occurrence of disturbances can therefore limit the recovery of microbial community composition and function in soils.
Ecosystems also experience temporal variability related to successional dynamics that could influence recovery and temporal stability by causing a change in baseline environmental conditions. Lakes, for example, often experience seasonal dynamics related to changes in temperature, light, and nutrient availability that could interfere with microbial resilience. Several studies that investigated the effect of extreme weather events (e.g., storms) or experimental water column mixing showed that microbial communities exhibit compositional and/or functional recovery within a few days or weeks (e.g., see references 45 and 92). However, continuous changes irrespective of recurring typhoons have also been shown in a study that analyzed metagenomes (93), suggesting that seasonal changes can “overrule” effects of disturbances, at least in some cases.
One large-scale ecosystem property of particular importance in relation to spatiotemporal dynamics is water residence time (94), which reflects the turnover time of water in a water body or mass. Water residence time is important to understand both the persistence and impact of an extrinsic pulse disturbance (e.g., inflow of pollutants, salinization, and eutrophication) as well as dispersal rates into local habitats (95) as microbes move with the flow of water through space. External inflows as well as water column mixing often lead to rather large-scale community mixing, i.e., coalescence (96), events in pelagic environments that lack comparison in soils. Coalescence can be seen both as a potential disturbance (e.g., a change in abiotic or biotic conditions) and as a dispersal event that may promote recovery if it rapidly reintroduces a locally extinct species from an external dispersal source. Dispersal might, moreover, lead to the immigration of species that replace local species which became extinct in response to the disturbance, hence fostering community turnover. This might be one possible explanation why compositional turnover has been found to be higher in aquatic systems than in soils (97).
Trophic structure and plant-microbe interactions.
One general difference between soil and pelagic microbial communities is the larger biomass of fungi in the former (81), which can have implications for resilience as discussed above. Moreover, in soils, interactions between primary producers, i.e., plants, and microbes are spatially decoupled, which can lead to indirect effects of aboveground disturbances that affect plants on microbial resilience. For example, some studies have shown that resistance and recovery of soil microbial biomass and community structure are more related to plant-induced changes than direct effects of drought disturbances on the soil microbiome (98, 99). Other results, however, suggest that direct disturbance effects on microbial physiology and soil chemical and physical conditions may be more important for changes in microbial community structure than changes in plant community structure predicted to result from climate change (100), so that there is a lack of consensus among studies. Finally, it has also been shown that differences in the resistance and recovery of different groups of microorganisms (bacteria and fungi) in response to drought depended on changes in plant community composition and physiology in response to the disturbances (101, 102).
In aquatic ecosystems, interactions between primary producers and heterotrophic bacteria are also commonly found (103, 104), and consequently, as in the case with plant-microbe interactions in soils, disturbances can affect the stability of the heterotrophic microbial community directly as well as indirectly. The latter might, for example, be found in cases where a disturbance selectively affects only the phytoplankton (e.g., changes in the light regime) and then propagates through the microbial food web (26, 75). Furthermore, it has also been shown that disturbance can change the interaction type between phytoplankton and heterotrophic bacteria from a symbiotic to a parasitic relationship when the algae become stressed (105). Due the “spatial co-occurrence” of different plankton groups, it remains, however, challenging to separate direct from indirect effects of disturbances on any particular “target organism group.”
Predictions for habitat-specific differences in resilience mechanisms.
Resilience in aquatic and terrestrial ecosystems is often studied separately (16), and the need to apply a more integrative approach has been highlighted (20). Several properties discussed in the previous sections that influence resilience differ between the edaphic and pelagic ecosystems, including greater diversity, dormancy (68, 82), and fungal abundance (81) but lower community turnover rates (106) in soils than in pelagic systems. From this we can, for example, hypothesize that community turnover should be more important for functional resilience in pelagic systems, whereas the insurance effect should be more important in soils, as their diversity is greater. Another prediction is that refuges offered by aggregates and biotic interactions play a more important role for soil than pelagic microbial resilience. Finally, we could expect that the contribution of dormancy to resilience, where microorganisms resuscitate from local seed banks, is more important in soils, whereas dispersal from external sources is more important in pelagic systems, at least when relatively large spatial scales are studied. Testing these admittedly bold predictions could be conducted using experimental studies that manipulate spatial heterogeneity and retention time (ideally both), as these are the two major overarching environmental characteristics that modulate effects of community and disturbance properties as well as assembly processes that influence microbial resilience. Moreover, experiments that compare the effects of the same type of disturbance on terrestrial and aquatic communities at the same time are needed. For example, Röhl et al. (107) compared the effects of short-term inundations/floodings on terrestrial and aquatic microeukaryote communities in freshwater mesocosms designed to represent river flood plains, where both ecosystems are intermittently connected by flooding intensity and frequency. They found that soil community composition was resilient, whereas aquatic community composition was not. These authors suggested that this was because particle suspension from soil to water resulting from flooding increased aquatic nutrient levels and caused a more persistent change in aquatic communities than in terrestrial communities. This study exemplifies that feedback between disturbance type and the properties of the microbial communities needs to be considered for a holistic understanding of resilience across ecosystems.
In summary, there is a growing consensus that microbial resilience is influenced by the interplay of a variety of properties and mechanisms that operate at different spatial and temporal scales and directly or indirectly affect different microbial community members. However, studies investigating the impact of disturbances on microbial communities seldom embraced this complexity and most often focused on one or two microbial kingdoms (mostly bacteria and/or fungi) and different ecosystems separately. This most likely conditioned a skewed and deformed understanding of how the same type of disturbance can affect the microbial communities as a whole (i.e., the blind men and the elephant paradox). Hence, there is a clear need for a more holistic assessment of microbial resilience that also includes other groups of the microbiota, including, e.g., viruses and protists. Currently, these limitations, together with differences in the experimental design between studies, make it difficult to decipher whether the observed differences in responses between studies were due to differences in physiology/traits of the microorganisms in the studied habitat or differences in the intensity/frequency/nature of the disturbance(s) applied, as this can also affect microbial resilience.
MULTIPLE DISTURBANCES AND MICROBIAL RESILIENCE
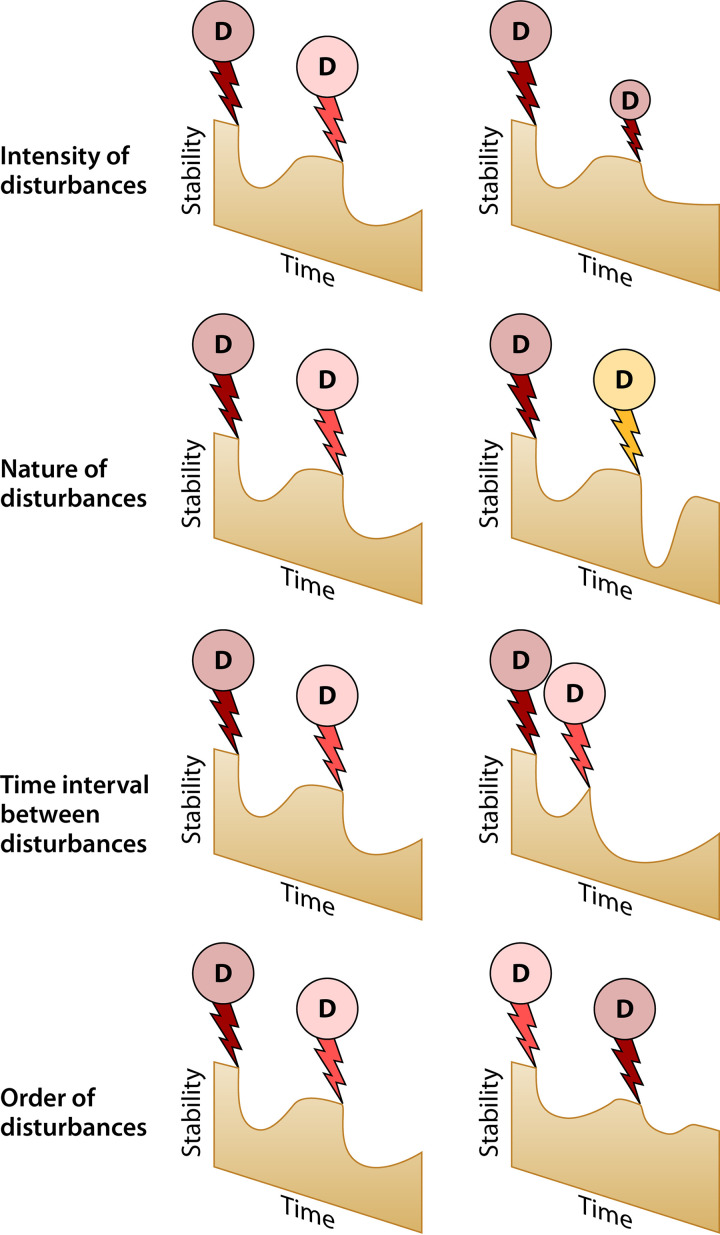
In the past years, we have witnessed more frequent extreme weather conditions due to climate change. Intensive anthropogenic activities are also increasing, subjecting ecosystems to disturbances not only of greater magnitude but also of increasing frequency, which is raising concerns about the capacity of ecosystems to absorb multiple disturbances. This is all the more important because there is potential for emerging interactions with the effects of subsequent disturbance events when ecosystems are exposed to several disturbances in a relatively short period of time (108). In other words, the legacy of an initial disturbance might alter the impact of the following disturbance, and the combined effects of multiple disturbances will be different than the sum of their individual effects. For example, meta-analyses revealed that the outcome of multiple disturbance interactions was typically nonadditive, i.e., combined effects could not be predicted based on single disturbance effects in approximately three-fourths of the studies (109, 110). Such interactive effects can have positive or negative feedbacks on resilience and trigger shifts to alternative stable states, therefore making predictions of ecosystem trajectories more uncertain (111–113). Modeling of soil microbial resilience also confirmed the importance of historical contingencies related to previous disturbances (114). A recent article by Rillig et al. (6) showed that the majority of studies investigated at the most two combined disturbances, with approximately 80% of studies focusing on only a single disturbance. Therefore, understanding the effects of multiple disturbances remains an important contemporary challenge for microbial ecologists (113). Addressing it requires disaggregating compounded disturbances into their constituent drivers, i.e., nature, intensity, frequency, and chronology (Fig. 5). The importance of disturbance intensity has been intensively studied for single disturbances and is tightly connected to the intermediate disturbance hypothesis (IDH) (115), which predicts that the highest biodiversity will occur at moderate disturbance (intensities and frequencies); therefore, disturbance intensity will only be discussed briefly here.
FIG 5.
Conceptual framework illustrating the importance of the inherent characteristics of disturbance sequences for microbial resilience (stability).
Nature of Compounded Disturbances
Microbial assemblages in all ecosystems undergo disturbances that can disrupt their structure and their physical environment. Odum (116) hypothesized that chronic (press or persistent) stress may have a different effect than acute (pulse or transient) stress that is quickly followed by recovery. This led a few years later to the distinction between pulse and press perturbations by Bender et al. (117), with a pulse disturbance being short-term and finite and a press disturbance being long lasting. Glasby and Underwood (118) also emphasized the importance of distinguishing the cause and the effect of disturbance, because, for example, a pulse disturbance might cause a press effect and vice versa. A literature survey by Shade et al. (16) showed a trend that microbial communities may be more resilient after a pulse disturbance than after a press disturbance and highlighted a knowledge gap in our understanding of the response of microbial communities to combined disturbances and, specifically, to those combining both pulses and press disturbances.
Multiple disturbances of the same nature.
It is commonly assumed that previous disturbance would result in an attenuated response of microbial communities to subsequent disturbances of the same nature. This could be due to enhanced physiological tolerance or adaptation, for example, based on modifications of the bacterial membrane or changes in the transcriptional regulation (119). Other possible mechanisms comprise community selection, where the disturbance acts as a filter, and long-term microbial evolution, where beneficial mutations (especially in the case of elevated mutation rates, i.e., hypermutability) confer tolerance to populations that have previously experienced the disturbance (120). On the other hand, a strong filtering effect of the disturbance can also have a merely ecological “knockout effect” and lead to loss of species, which could decrease resilience to the subsequent disturbance.
Since drought periods are predicted to intensify with global warming, the response to repeated drought pulse disturbances has been widely investigated in soil. Drought constrains substrate diffusion, leading to resource limitation and reduced microbial abundance and activity, and possibly induces dormancy (104, 121, 122). Drying also induces various protection mechanisms such as biofilm formation or osmolyte accumulation to reduce the internal water potential to avoid dehydration. Upon rewetting, microbes must rapidly release the accumulated osmolytes to maintain osmotic equilibrium. Several studies showed that exposure to repeated drought disturbance can increase resistance, whether of microbial community composition (123), phylogenetic diversity (124), or growth and activity (125, 126). Similar responses to repeated heat disturbances have also been observed (127). However, there are also studies that show that exposure to repeated disturbances does not lead to adaptation of the microbial community. For example, exposure of lake sediments and paddy soil to a new desiccation event did not show that disturbance history had an effect on the response of methanotrophs (128). Thus, a legacy effect of repeated drought disturbances is not consistently found and appears to differ depending on land use and soil type (129–132). Moreover, different responses are also found between different groups of organisms and functional responses. For example, previous drought reduced the resilience of bacteria and Prostigmata after a second drought, whereas it increased resilience of Mesostigmata, Oribatida, and Astigmata (101). Kaisermann et al. (133) found that respiration and dissolved organic carbon and ammonium concentrations but not bacterial and fungal community composition were affected by drought history in grassland soil. In contrast, shifts in bacterial community composition but not in taxonomic diversity and richness were observed after repeated dry-wet cycles (129).
A typical example of a multiple-press disturbance that is ubiquitously present in terrestrial and freshwater ecosystems is contamination by toxic compounds, such as heavy metals, due to both natural and anthropogenic releases from agriculture, urbanization, mining, and industry. Microbes have evolved various mechanisms to counteract the toxic effects of heavy metals, and heavy-metal contamination is therefore a good example of a press disturbance that should lead to adaptation/tolerance. For example, Tlili et al. (134) showed that resistance to copper, zinc, or arsenic increased when preexposing biofilm communities to the same metal. They also found clear evidence of coresistance between different metals, and both phototrophic and heterotrophic communities that were preexposed to copper were more resistant to a subsequent zinc disturbance than an undisturbed community and vice versa (134). Similarly, in soil microcosms, initial exposure to a heavy metal increased the resistance and resilience of microbial activities to subsequent disturbance by a different contaminating metal (135, 136). Coresistance between different metals, however, does not always occur (134). Moreover, whether the metal disturbance is due to an abrupt or gradual increase in soil heavy-metal concentration has been shown to be important for microbial communities only in the short term. Thus, gradual and abrupt copper disturbance led to initial differences in bacterial community composition and respiration, but communities in both systems appeared to converge once copper concentrations were equal (137).
Multiple disturbances of different natures.
While compounded disturbances of the same nature are predicted to lead to some adaptation, microbial communities recovering from an initial perturbation are predicted to be more susceptible to a novel disturbance of a different nature (127). Calderón et al. (5) showed that exposure of soil microcosms to sequences comprising three different types of pulse disturbances (e.g., freeze-thaw, heat-drought, and anoxia) led to lower resilience in bacterial community structure and soil functions than in microcosms exposed to the same repeated disturbance. Similarly, exposure of lake sediments and paddy soil with contrasted desiccation history, to either desiccation alone or combined with a heat shock, led to differences in methanotrophic community composition and activity, with a lower and slower recovery after the heat shock in the case of the lake sediment (128). Initial exposure to a press disturbance such as heavy-metal contamination is also likely to negatively affect the response of microbial communities to a subsequent novel disturbance, since exposure to contaminants requires reallocation of more energy to detoxification, damage repair, and tolerance, which therefore can make it harder for microbes to cope with an additional disturbance. Thus, exposure to heavy-metal contamination reduced microbial respiration and led to a larger shift in bacterial and archaeal community composition in two soils subjected to dry-wet cycles (138, 139). On the other hand, support also exists for the prediction that communities preexposed to disturbance are more resilient to a new type of disturbance, due to cotolerance to multiple stressors (140) or acquired stress resistance (141), where the previous disturbances activate different cellular stress response mechanisms that “prepare” them for additional disturbances. In one example, experimental warming differently increased the functional tolerance of heterotrophic communities in freshwater ecosystems to copper disturbance, even though the response differed between functions (142). Another study showed that an initial pH change increased the resistance of cell-specific extracellular enzyme activities and the engineering resilience of cell-specific bacterial production in bacterial freshwater communities exposed to a salt disturbance, while community composition was not affected (143). Likewise, exposure to a pH disturbance of a freshwater bacterial community that was previously exposed to sequences of temperature pulse disturbances of different intensities increased the resistance and recovery of bacterial carbon production and extracellular enzyme activities but did not affect composition (144).
The effects of the combination of multiple disturbances on soil functions and microbial diversity was recently investigated by Rillig et al. (6). Increasing the number of simultaneous disturbances from 1 to 10 caused increasing directional changes in the response of soil fungal diversity as well as soil processes. Interestingly, some response variables were resistant until 8 or more disturbances were combined, which illustrates how challenging it is to predict the effects of multiple disturbances, especially when sequences of pulse and press disturbances are mixed.
Intensity and Frequency of Disturbances
Disturbances can cause secondary successions of microbial communities, including shifts in their composition and diversity. This also means that a new disturbance may affect communities differently depending on their autogenic succession phase, which can be affected by both the intensity and frequency of recurring disturbances. The time lag between disturbances may also determine whether or not there is sufficient time for dispersal and colonization of the disturbed local ecosystem from the regional species pool. Whether a new disturbance event occurs during the recovery from a previous disturbance may therefore modulate the microbiome’s response to a new disturbance. Jurburg et al. (145), for example, showed that secondary succession after disturbance can affect bacterial communities in soil for at least 25 days, during which communities might be more susceptible to a new disturbance. Another study highlighted that disturbance intensity can influence compositional and functional resilience in response to recurring temperature pulse disturbances applied at the same frequency (144). Repeated exposure to stronger temperature pulses attenuated decreases in bacterial richness and evenness and led to differential functional responses depending on the type of function: while resistance and recovery of bacterial abundance and carbon production tended to decrease after several disturbances, the activities of extracellular enzymes increased over time as the community accumulated a disturbance history. Hence, this example shows that microbial resilience in response to new disturbances not only depends on disturbance intensity but can also differ depending on the response variable.
How the time interval between disturbances (Fig. 5) moderates ecosystem responses likely also depends on the nature of the disturbance (Fig. 5). For compounded disturbances of different natures, a longer interval between disturbances may help microbial communities to cope with the new disturbance, while longer intervals between similar disturbances may produce the opposite pattern (145). In support of the latter, Seneviratne and Marschner (146) found increased differences in soil respiration and mineral nitrogen availability compared to those in the control with increasing time interval between two heating events. Resilience may in fact reach a tipping point when disturbances increase in frequency (147), as has been shown in freshwater rock pools where there were stronger effects on bacterial community composition with increasing frequencies and reduced time intervals of salt disturbance (63). In many studies, the time interval between disturbances is negatively related to the number of disturbance events (147), and overall, surprisingly few studies have examined the importance of the time interval between disturbances on microbial resilience independently of disturbance frequency, which highlights a research gap to be addressed in future studies.
Chronological Order of Disturbances
Experimental approaches that manipulate disturbances in a factorial manner to rigorously test for effects of disturbance chronology are even rarer than intensity and time interval studies. Generally, disturbance B occurring after disturbance A might place a microbial community in a different position on its adaptive trajectory compared to that for the situation where disturbance A occurs after B (Fig. 4). This could be caused by various higher order interactions such as, for example, changes in community composition or physiology. Calderón et al. (5) examined whether the responses of soil microbial community composition and function differed when subjected to series comprising the same three pulse disturbances (freeze-thaw, heat, and anoxia) that were applied at the same frequency and intensity but in a different chronological order. The disturbance chronology affected bacterial community composition and determined the aggregated impact on ecosystem properties and functions (Fig. 6). Disturbance chronology had a large impact on several ecosystem properties such as NH4+ concentration and abundance of various N-cycling microbial communities as well as bacterial phylogenetic diversity and richness. Approximately 30% of the observed variance in the aggregated ecosystem impact was linked to shifts in bacterial community composition (5), whereas the importance of physiological plasticity or adaptation remained unknown.
FIG 6.
Aggregated impact of compounded disturbances with alternative chronologies on ecosystem properties and functions 3 weeks (A) and 10 weeks (B) after the last disturbance cycle. The “ecosystem aggregated impact” was calculated as the sum of the absolute value of Hedges’ g for all studied variables. Means with the same lowercase letter (a, b, or c) are not significantly different; bars represent standard errors. Adapted from reference 5.
In summary, there is a large disparity in results between different studies, which often, but not always, show that disturbance legacies are important for the compositional and functional resilience of microbial communities. This lack of consistency might have various reasons. First, biotic and abiotic differences in the studied ecosystems in relation to the underlying mechanisms highlighted in the previous section (see also Shade et al. [16]) might explain diverging results. Moreover, inconsistent results among studies may also simply reflect differences in disturbance properties, i.e., the frequency, intensity, and/or chronology of the disturbances, which highlights the need to explicitly consider all inherent characteristics of a disturbance sequence. The role of biotic interactions such as mutualisms or competition between microbial species has also been largely overlooked in microbial resilience studies and is likely even more important in the case of multiple disturbances (see Fig. 4). Biotic interactions can potentially also modulate microbial community resilience by determining the strength of priority effects. Thus, the specific order by which microbial species arrive by dispersal or resuscitate from dormancy may influence the establishment success of later arriving/resuscitating species and therefore community resilience (e.g., Svoboda et al. [148]).
CONCLUSIONS AND FUTURE DIRECTIONS
Undoubtedly, addressing how disturbances affect microbial communities has received increasing interest during the last 2 decades. Much has been learned about the responses of the microbiota to a large variety of disturbances in diverse ecosystems. Conceptual, theoretical, and modeling frameworks have also been proposed to understand the mechanisms driving these changes in microbial communities facing global change factors or anthropogenic contamination. It is clear that disturbances have detrimental effects on the diversity and composition of microbial communities that can impair their ability to perform crucial ecosystem functions involved in biogeochemical cycling. As such, the recovery of microbial communities from disturbance is a key part in the fate of multifunctionality and the delivery of ecosystem services. Unique features of microbes, e.g., gene transfer, dormancy, dispersal abilities, or speciation rate, underpinning microbial community assembly are intrinsically linked to microbial resilience. Comparing microbial resilience across ecosystems allowed us to identify specific properties that differ along scales of spatial and temporal heterogeneity. This might be used to establish a general conceptual framework to gain a holistic understanding about microbial resilience across ecosystems and that further reflects the scale-dependency of resilience mechanisms and disturbance properties in time and space. Moreover, the indirect effects of disturbances on ecological interactions between microorganisms belonging to different kingdoms (viruses, bacteria, archaea, fungi, protists, etc.) are only beginning to be understood. The rapid advances in high-throughput sequencing, database coverage, and network inference algorithms are now transforming the field (149) and will allow us to address the importance of community composition across domains, rare taxa, dispersal, or biotic interactions as well as the identity of the key traits driving microbial resilience.
Although important insights have been obtained, our ability to predict the direction and the magnitude of microbial and ecosystem responses to global change has not really improved. This task is even more challenging considering that the large majority of studies focused on one or two global change drivers, while ecosystems are increasingly exposed to multiple disturbances, which are not only more frequent but also have more different natures. Although they work in unison, the effects of the disturbance nature and, to a lesser extent, intensity have been widely investigated, while data on disturbance frequency and chronology are underrepresented. A more detailed examination of the interplay between the different inherent characteristics should be the next step to improve our understanding and ability to predict the impact of multiple disturbances. Disentangling and quantifying the relative importance of the main disturbance characteristics can be challenging due to the explosion of factors that need to be considered if assessed in full factorial experiments. Another key consideration for future studies is to also measure responses at different time scales to be able to properly capture the resistance, recovery, engineering resilience, and other resilience and stability descriptors of multiple organisms and habitats in response to multiple disturbances.
Hence, microbial ecologists are facing a key challenge to include all this complexity in future designs of experimental and observational studies, but there are several promising ways ahead to do so. First, innovative experimental designs can be applied. For example, Rillig et al. (6) used an experimental design based on approaches widely used in studies that address biodiversity ecosystem functioning relationships, where a species pool is selected at random for each level of diversity, which prevents the inflation of treatments (150). In their case, they randomly selected global change drivers, rather than species, to show that increasing the number of simultaneous global change factors resulted in increasing directional changes in microbial processes and communities. Second, the application of a minimum reporting standard in disturbance investigations to promote consensus and compliance among researchers carrying out future studies would be another important step in improving our ability to integrate invaluable information collected from different sources (151). Third, related to this, coordinated experiments that apply the same standardized operating procedures, including experimental setup, treatments, and analysis methods, would be highly valuable as well. This approach is, for example, successfully used in the Nutrient Network (www.nutnet.org) and Zostera Experimental Network (http://zenscience.org/), which focus of herbaceous grassland and eelgrass communities, respectively. Applying similar collaborative approaches also offers great promise to study the resilience of microbes across spatial and temporal scales.
ACKNOWLEDGMENTS
The efforts in preparing this review were supported by funding from the UBFC-ISITE senior fellowship (grant RA19016.AEC.IS to L.P.).
We thank Aymé Spor for helpful discussion and valuable comments on the manuscript.
Biographies

Laurent Philippot is director of research at the INRAE in the Agroecology Department, Dijon, France. He received a Ph.D. from the University Claude Bernard, Lyon, in 1997 and did a sabbatical at the Georgia University of Technology, Atlanta, as well at the Swedish University of Agricultural Science, Uppsala. He is interested in bridging microbial community ecology, microbial processes, and ecosystem functioning using a trait-centered approach. He is editor of The ISME Journal and editorial board member of FEMS Microbiology Ecology as well as Applied and Environmental Microbiology.

Bryan S. Griffiths has recently retired as professor of soil ecology from the Department of Agriculture, Horticulture and Engineering Sciences at SRUC (Scotland’s Rural College) in Edinburgh. After attaining his Ph.D. from the University of Dundee in 1982, he went on to work at the Macaulay Institute for Soil Research in Aberdeen and the Scottish Crop Research Institute in Dundee. In 2008, Bryan became the Science Foundation Ireland professor of soil science, based at the Teagasc environmental research center in Wexford before moving finally to SRUC. He has a long-standing interest in microbial interactions, especially between microfauna (nematodes and protozoa) and microbes and plants. Currently a field editor for the European Journal of Soil Biology, he has served on the editorial board of Pedobiologia, Applied Soil Ecology, and Biology and Fertility of Soils.

Silke Langenheder is a professor at the Department of Ecology and Genetics at Uppsala University, Sweden, and Director of the Erken Laboratory, a limnological field station which is part of the Swedish Infrastructure of Ecosystem sciences (SITES). She received her Ph.D. in 2005 from Uppsala University and spent 2 years as a post doc at the University of Aberdeen. She is interested in the processes that regulate microbial diversity across time and spaces and the implications that has for ecosystem functioning and stability. She is currently a Subject Editor for OIKOS and editorial board member of Environmental Microbiology.
REFERENCES
- 1.FAO, ITPS. 2015. Status of the world’s soil resources: main report. FAO, ITPS, Rome, Italy. [Google Scholar]
- 2.Payá Pérez A, Rodríguez Eugenio N. 2018. Status of local soil contamination in Europe revision of the indicator “Progress in the management contaminated sites in Europe”. European Commission, Joint Research Centre, Ispra, Italy. [Google Scholar]
- 3.Korpinen S, Klančnik K, Peterlin M, Nurmi M, Laamanen L, Zupančič G, Murray C, Harvey T, Andersen JH, Zenetos A, Stein U, Tunesi L, Abhold K, Piet G, Kallenbach E, Agnesi S, Bolman B, Vaughan D, Reker J, Royo Gelabert E. 2020. ETC/ICM technical report 4/2019: multiple pressures and their combined effects in Europe’s seas. European Topic Centre on Inland, Coastal and Marine Waters, EEA, Copenhagen, Denmark. [Google Scholar]
- 4.Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services, IPBES. 2019. Summary for policymakers of the global assessment report on biodiversity and ecosystem services. IPBES, Bonn, Germany. [Google Scholar]
- 5.Calderón K, Philippot L, Bizouard F, Breuil M-C, Bru D, Spor A. 2018. Compounded disturbance chronology modulates the resilience of soil microbial communities and N-cycle related functions. Front Microbiol 9:2721. 10.3389/fmicb.2018.02721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rillig MC, Ryo M, Lehmann A, Aguilar-Trigueros CA, Buchert S, Wulf A, Iwasaki A, Roy J, Yang G. 2019. The role of multiple global change factors in driving soil functions and microbial biodiversity. Science 366:886–890. 10.1126/science.aay2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Glöckner FO, Stal LJ, Sandaa R-A, Gasol JM, O’Gara F, Hernandez F, Labrenz M, Stoica E, Varela MM, Bordalo A, Pitta P. 2012. Marine Board-ESF position paper 17. In Calewaert JB, McDonough N (ed), Marine microbial diversity and its role in ecosystem functioning and environmental change. Marine Board-ESF, Ostend, Belgium. [Google Scholar]
- 8.Finlay BJ, Maberly SC, Cooper JI. 1997. Microbial diversity and ecosystem function. Oikos 80:209–213. 10.2307/3546587. [DOI] [Google Scholar]
- 9.Bardgett RD, van der Putten WH. 2014. Belowground biodiversity and ecosystem functioning. Nature 515:505–511. 10.1038/nature13855. [DOI] [PubMed] [Google Scholar]
- 10.Philippot L, Raaijmakers JM, Lemanceau P, van der Putten WH. 2013. Going back to the roots: the microbial ecology of the rhizosphere. Nat Rev Microbiol 11:789–799. 10.1038/nrmicro3109. [DOI] [PubMed] [Google Scholar]
- 11.Handa IT, Aerts R, Berendse F, Berg MP, Bruder A, Butenschoen O, Chauvet E, Gessner MO, Jabiol J, Makkonen M, McKie BG, Malmqvist B, Peeters ETHM, Scheu S, Schmid B, van Ruijven J, Vos VCA, Hättenschwiler S. 2014. Consequences of biodiversity loss for litter decomposition across biomes. Nature 509:218–221. 10.1038/nature13247. [DOI] [PubMed] [Google Scholar]
- 12.Wagg C, Bender SF, Widmer F, van der Heijden MGA. 2014. Soil biodiversity and soil community composition determine ecosystem multifunctionality. Proc Natl Acad Sci U S A 111:5266–5270. 10.1073/pnas.1320054111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Delgado-Baquerizo M, Maestre FT, Reich PB, Jeffries TC, Gaitan JJ, Encinar D, Berdugo M, Campbell CD, Singh BK. 2016. Microbial diversity drives multifunctionality in terrestrial ecosystems. Nat Commun 7:10541. 10.1038/ncomms10541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lefcheck JS, Byrnes JEK, Isbell F, Gamfeldt L, Griffin JN, Eisenhauer N, Hensel MJS, Hector A, Cardinale BJ, Duffy JE. 2015. Biodiversity enhances ecosystem multifunctionality across trophic levels and habitats. Nat Commun 6:6936. 10.1038/ncomms7936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Allison SD, Martiny JBH. 2008. Resistance, resilience, and redundancy in microbial communities. Proc Natl Acad Sci U S A 105:11512–11519. 10.1073/pnas.0801925105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shade A, Peter H, Allison SD, Baho DL, Berga M, Bürgmann H, Huber DH, Langenheder S, Lennon JT, Martiny JBH, Matulich KL, Schmidt TM, Handelsman J. 2012. Fundamentals of Microbial Community Resistance and Resilience. Front Microbiol 3:417. 10.3389/fmicb.2012.00417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Griffiths BS, Philippot L. 2013. Insights into the resistance and resilience of the soil microbial community. FEMS Microbiol Rev 37:112–129. 10.1111/j.1574-6976.2012.00343.x. [DOI] [PubMed] [Google Scholar]
- 18.Angeler DG, Allen CR. 2016. Quantifying resilience. J Appl Ecol 53:617–624. 10.1111/1365-2664.12649. [DOI] [Google Scholar]
- 19.Hodgson D, McDonald JL, Hosken DJ. 2015. What do you mean, ‘resilient’? Trends Ecol Evol 30:503–506. 10.1016/j.tree.2015.06.010. [DOI] [PubMed] [Google Scholar]
- 20.Ingrisch J, Bahn M. 2018. Towards a comparable quantification of resilience. Trends Ecol Evol 33:251–259. 10.1016/j.tree.2018.01.013. [DOI] [PubMed] [Google Scholar]
- 21.Oliver TH, Heard MS, Isaac NJB, Roy DB, Procter D, Eigenbrod F, Freckleton R, Hector A, Orme CDL, Petchey OL, Proença V, Raffaelli D, Suttle KB, Mace GM, Martín-López B, Woodcock BA, Bullock JM. 2015. Biodiversity and resilience of ecosystem functions. Trends Ecol Evol 30:673–684. 10.1016/j.tree.2015.08.009. [DOI] [PubMed] [Google Scholar]
- 22.Mori AS. 2016. Resilience in the studies of biodiversity–ecosystem functioning. Trends Ecol Evol 31:87–89. 10.1016/j.tree.2015.12.010. [DOI] [PubMed] [Google Scholar]
- 23.Song H-S, Renslow RS, Fredrickson JK, Lindemann SR. 2015. Integrating ecological and engineering concepts of resilience in microbial communities. Front Microbiol 6:1298. 10.3389/fmicb.2015.01298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Todman LC, Fraser FC, Corstanje R, Deeks LK, Harris JA, Pawlett M, Ritz K, Whitmore AP. 2016. Defining and quantifying the resilience of responses to disturbance: a conceptual and modelling approach from soil science. Sci Rep 6:28426. 10.1038/srep28426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Donohue I, Petchey OL, Montoya JM, Jackson AL, McNally L, Viana M, Healy K, Lurgi M, O'Connor NE, Emmerson MC. 2013. On the dimensionality of ecological stability. Ecol Lett 16:421–429. 10.1111/ele.12086. [DOI] [PubMed] [Google Scholar]
- 26.Hillebrand H, Langenheder S, Lebret K, Lindström E, Östman Ö, Striebel M. 2018. Decomposing multiple dimensions of stability in global change experiments. Ecol Lett 21:21–30. 10.1111/ele.12867. [DOI] [PubMed] [Google Scholar]
- 27.Schaeffer A, Amelung W, Hollert H, Kaestner M, Kandeler E, Kruse J, Miltner A, Ottermanns R, Pagel H, Peth S, Poll C, Rambold G, Schloter M, Schulz S, Streck T, Roß-Nickoll M. 2016. The impact of chemical pollution on the resilience of soils under multiple stresses: a conceptual framework for future research. Sci Total Environ 568:1076–1085. 10.1016/j.scitotenv.2016.06.161. [DOI] [PubMed] [Google Scholar]
- 28.Wolkovich EM, Cook BI, McLauchlan KK, Davies TJ. 2014. Temporal ecology in the Anthropocene. Ecol Lett 17:1365–1379. 10.1111/ele.12353. [DOI] [PubMed] [Google Scholar]
- 29.Vellend M. 2010. Conceptual synthesis in community ecology. Q Rev Biol 85:183–206. 10.1086/652373. [DOI] [PubMed] [Google Scholar]
- 30.Gubry-Rangin C, Kratsch C, Williams TA, McHardy AC, Embley TM, Prosser JI, Macqueen DJ. 2015. Coupling of diversification and pH adaptation during the evolution of terrestrial Thaumarchaeota. Proc Natl Acad Sci U S A 112:9370–9375. 10.1073/pnas.1419329112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boles BR, Thoendel M, Singh PK. 2004. Self-generated diversity produces “insurance effects” in biofilm communities. Proc Natl Acad Sci U S A 101:16630–16635. 10.1073/pnas.0407460101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yachi S, Loreau M. 1999. Biodiversity and ecosystem productivity in a fluctuating environment: the insurance hypothesis. Proc Natl Acad Sci U S A 96:1463–1468. 10.1073/pnas.96.4.1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang Q-G, Lu H-S, Buckling A. 2018. Temperature drives diversification in a model adaptive radiation. Proc Biol Sci 285:20181515. 10.1098/rspb.2018.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Heuer H, Smalla K. 2012. Plasmids foster diversification and adaptation of bacterial populations in soil. FEMS Microbiol Rev 36:1083–1104. 10.1111/j.1574-6976.2012.00337.x. [DOI] [PubMed] [Google Scholar]
- 35.Rahube TO, Viana LS, Koraimann G, Yost CK. 2014. Characterization and comparative analysis of antibiotic resistance plasmids isolated from a wastewater treatment plant. Front Microbiol 5:558. 10.3389/fmicb.2014.00558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hemme CL, Green SJ, Rishishwar L, Prakash O, Pettenato A, Chakraborty R, Deutschbauer AM, Van Nostrand JD, Wu L, He Z, Jordan IK, Hazen TC, Arkin AP, Kostka JE, Zhou J. 2016. Lateral gene transfer in a heavy metal-contaminated-groundwater microbial community. mBio 7:e02234-15. 10.1128/mBio.02234-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tesson SVM, Okamura B, Dudaniec RY, Vyverman W, Löndahl J, Rushing C, Valentini A, Green AJ. 2015. Integrating microorganism and macroorganism dispersal: modes, techniques and challenges with particular focus on co-dispersal. Écoscience 22:109–124. 10.1080/11956860.2016.1148458. [DOI] [Google Scholar]
- 38.Székely AJ, Langenheder S. 2017. Dispersal timing and drought history influence the response of bacterioplankton to drying–rewetting stress. ISME J 11:1764–1776. 10.1038/ismej.2017.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sorensen JW, Shade A. 2020. Dormancy dynamics and dispersal contribute to soil microbiome resilience. Philos Trans R Soc Lond B Biol Sci 375:20190255. 10.1098/rstb.2019.0255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shen D, Langenheder S, Jürgens K. 2018. Dispersal modifies the diversity and composition of active bacterial communities in response to a salinity disturbance. Front Microbiol 9:2188. 10.3389/fmicb.2018.02188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van Elsas JD, Chiurazzi M, Mallon CA, Elhottova D, Kristufek V, Salles JF. 2012. Microbial diversity determines the invasion of soil by a bacterial pathogen. Proc Natl Acad Sci U S A 109:1159–1164. 10.1073/pnas.1109326109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Spor A, Camargo ARO, Bru D, Gaba S, Garmyn D, Gal L, Piveteau P. 2020. Habitat disturbances modulate the barrier effect of resident soil microbiota on Listeria monocytogenes invasion success. Front Microbiol 11:927. 10.3389/fmicb.2020.00927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Calderón K, Spor A, Breuil M-C, Bru D, Bizouard F, Violle C, Barnard RL, Philippot L. 2017. Effectiveness of ecological rescue for altered soil microbial communities and functions. ISME J 11:272–283. 10.1038/ismej.2016.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Klappenbach JA, Dunbar JM, Schmidt TM. 2000. rRNA operon copy number reflects ecological strategies of bacteria. Appl Environ Microbiol 66:1328–1333. 10.1128/aem.66.4.1328-1333.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jones SE, Chiu C-Y, Kratz TK, Wu J-T, Shade A, McMahon KD. 2008. Typhoons initiate predictable change in aquatic bacterial communities. Limnol Oceanogr 53:1319–1326. 10.4319/lo.2008.53.4.1319. [DOI] [Google Scholar]
- 46.Kurm V, Geisen S, Gera Hol WH. 2019. A low proportion of rare bacterial taxa responds to abiotic changes compared with dominant taxa: weak response of rare bacteria to abiotic change. Environ Microbiol 21:750–758. 10.1111/1462-2920.14492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Simon M, López-García P, Deschamps P, Restoux G, Bertolino P, Moreira D, Jardillier L. 2016. Resilience of freshwater communities of small microbial eukaryotes undergoing severe drought events. Front Microbiol 7:812. 10.3389/fmicb.2016.00812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.MacArthur RH, Wilson EO. 2001. The theory of island biogeography. Princeton University Press, Princeton, NJ. [Google Scholar]
- 49.Frenk S, Hadar Y, Minz D. 2017. Quality of irrigation water affects soil functionality and bacterial community stability in response to heat disturbance. Appl Environ Microbiol 84:e02087-17. 10.1128/AEM.02087-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.van der Voort M, Kempenaar M, van Driel M, Raaijmakers JM, Mendes R. 2016. Impact of soil heat on reassembly of bacterial communities in the rhizosphere microbiome and plant disease suppression. Ecol Lett 19:375–382. 10.1111/ele.12567. [DOI] [PubMed] [Google Scholar]
- 51.de Vries FT, Shade A. 2013. Controls on soil microbial community stability under climate change. Front Microbiol 4:265. 10.3389/fmicb.2013.00265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Roller BRK, Stoddard SF, Schmidt TM. 2016. Exploiting rRNA operon copy number to investigate bacterial reproductive strategies. Nat Microbiol 1:16160. 10.1038/nmicrobiol.2016.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kearns PJ, Shade A. 2018. Trait-based patterns of microbial dynamics in dormancy potential and heterotrophic strategy: case studies of resource-based and post-press succession. ISME J 12:2575–2581. 10.1038/s41396-018-0194-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schwarz B, Barnes AD, Thakur MP, Brose U, Ciobanu M, Reich PB, Rich RL, Rosenbaum B, Stefanski A, Eisenhauer N. 2017. Warming alters energetic structure and function but not resilience of soil food webs. Nat Clim Chang 7:895–900. 10.1038/s41558-017-0002-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Thakur MP, Del Real IM, Cesarz S, Steinauer K, Reich PB, Hobbie S, Ciobanu M, Rich R, Worm K, Eisenhauer N. 2019. Soil microbial, nematode, and enzymatic responses to elevated CO2, N fertilization, warming, and reduced precipitation. Soil Biol Biochem 135:184–193. 10.1016/j.soilbio.2019.04.020. [DOI] [Google Scholar]
- 56.de Vries FT, Griffiths RI, Bailey M, Craig H, Girlanda M, Gweon HS, Hallin S, Kaisermann A, Keith AM, Kretzschmar M, Lemanceau P, Lumini E, Mason KE, Oliver A, Ostle N, Prosser JI, Thion C, Thomson B, Bardgett RD. 2018. Soil bacterial networks are less stable under drought than fungal networks. Nat Commun 9:3033. 10.1038/s41467-018-05516-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Meisner A, Jacquiod S, Snoek BL, ten Hooven FC, van der Putten WH. 2018. Drought legacy effects on the composition of soil fungal and prokaryote communities. Front Microbiol 9:294. 10.3389/fmicb.2018.00294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.van der Plas F. 2019. Biodiversity and ecosystem functioning in naturally assembled communities. Biol Rev 94:1220–1245. 10.1111/brv.12499. [DOI] [PubMed] [Google Scholar]
- 59.Hallin S, Welsh A, Stenström J, Hallet S, Enwall K, Bru D, Philippot L. 2012. Soil functional operating range linked to microbial biodiversity and community composition using denitrifiers as model guild. PLoS One 7:e51962. 10.1371/journal.pone.0051962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Orwin KH, Dickie IA, Wood JR, Bonner KI, Holdaway RJ. 2016. Soil microbial community structure explains the resistance of respiration to a dry-rewet cycle, but not soil functioning under static conditions. Funct Ecol 30:1430–1439. 10.1111/1365-2435.12610. [DOI] [Google Scholar]
- 61.Delgado-Baquerizo M, Eldridge DJ, Ochoa V, Gozalo B, Singh BK, Maestre FT. 2017. Soil microbial communities drive the resistance of ecosystem multifunctionality to global change in drylands across the globe. Ecol Lett 20:1295–1305. 10.1111/ele.12826. [DOI] [PubMed] [Google Scholar]
- 62.Koch BJ, McHugh TA, Hayer M, Schwartz E, Blazewicz SJ, Dijkstra P, Gestel N, Marks JC, Mau RL, Morrissey EM, Pett‐Ridge J, Hungate BA. 2018. Estimating taxon‐specific population dynamics in diverse microbial communities. Ecosphere 9:e02090. 10.1002/ecs2.2090. [DOI] [Google Scholar]
- 63.Berga M, Székely AJ, Langenheder S. 2012. Effects of disturbance intensity and frequency on bacterial community composition and function. PLoS One 7:e36959. 10.1371/journal.pone.0036959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Carini P, Marsden PJ, Leff JW, Morgan EE, Strickland MS, Fierer N. 2016. Relic DNA is abundant in soil and obscures estimates of soil microbial diversity. Nat Microbiol 2:16242. 10.1038/nmicrobiol.2016.242. [DOI] [PubMed] [Google Scholar]
- 65.Foster KR, Bell T. 2012. Competition, not cooperation, dominates interactions among culturable microbial species. Curr Biol 22:1845–1850. 10.1016/j.cub.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 66.West SA, Cooper GA. 2016. Division of labour in microorganisms: an evolutionary perspective. Nat Rev Microbiol 14:716–723. 10.1038/nrmicro.2016.111. [DOI] [PubMed] [Google Scholar]
- 67.Rillig MC, Muller LA, Lehmann A. 2017. Soil aggregates as massively concurrent evolutionary incubators. ISME J 11:1943–1948. 10.1038/ismej.2017.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tecon R, Or D. 2017. Biophysical processes supporting the diversity of microbial life in soil. FEMS Microbiol Rev 41:599–623. 10.1093/femsre/fux039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ebenman B, Jonsson T. 2005. Using community viability analysis to identify fragile systems and keystone species. Trends Ecol Evol 20:568–575. 10.1016/j.tree.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 70.Röttjers L, Faust K. 2018. From hairballs to hypotheses–biological insights from microbial networks. FEMS Microbiol Rev 42:761–780. 10.1093/femsre/fuy030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.MacArthur R. 1955. Fluctuations of animal populations and a measure of community stability. Ecology 36:533–536. 10.2307/1929601. [DOI] [Google Scholar]
- 72.May RM. 1972. Will a large complex system be stable? Nature 238:413–414. 10.1038/238413a0. [DOI] [PubMed] [Google Scholar]
- 73.Thebault E, Fontaine C. 2010. Stability of ecological communities and the architecture of mutualistic and trophic networks. Science 329:853–856. 10.1126/science.1188321. [DOI] [PubMed] [Google Scholar]
- 74.Bissett A, Brown MV, Siciliano SD, Thrall PH. 2013. Microbial community responses to anthropogenically induced environmental change: towards a systems approach. Ecol Lett 16:128–139. 10.1111/ele.12109. [DOI] [PubMed] [Google Scholar]
- 75.Hunt DE, Ward CS. 2015. A network-based approach to disturbance transmission through microbial interactions. Front Microbiol 6:1182. 10.3389/fmicb.2015.01182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Puche E, Rojo C, Rodrigo MA. 2020. Multi-interaction network performance under global change: a shallow ecosystem experimental simulation. Hydrobiologia 847:3549–3569. 10.1007/s10750-020-04359-y. [DOI] [Google Scholar]
- 77.MacLaren RG, Cameron KC. 2002. Soil science: sustainable production and environmental protection, 2nd ed. Oxford University Press, Oxford, United Kingdom. [Google Scholar]
- 78.Killham K. 1994. Soil ecology. Cambridge University Press, Cambridge, United Kingdom. [Google Scholar]
- 79.Bachmann J, Heimbach T, Hassenrück C, Kopprio GA, Iversen MH, Grossart HP, Gärdes A. 2018. Environmental drivers of free-living vs. particle-attached bacterial community composition in the Mauritania upwelling system. Front Microbiol 9:2836. 10.3389/fmicb.2018.02836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Vos M, Wolf AB, Jennings SJ, Kowalchuk GA. 2013. Micro‐scale determinants of bacterial diversity in soil. FEMS Microbiol Rev 37:936–954. 10.1111/1574-6976.12023. [DOI] [PubMed] [Google Scholar]
- 81.Kirchman DL. 2018. Processes in microbial ecology, 2nd edition. Oxford University Press, Oxford, United Kingdom. [Google Scholar]
- 82.Lennon JT, Jones SE. 2011. Microbial seed banks: the ecological and evolutionary implications of dormancy. Nat Rev Microbiol 9:119–130. 10.1038/nrmicro2504. [DOI] [PubMed] [Google Scholar]
- 83.Baho DL, Peter H, Tranvik LJ. 2012. Resistance and resilience of microbial communities–temporal and spatial insurance against perturbations. Environ Microbiol 14:2283–2292. 10.1111/j.1462-2920.2012.02754.x. [DOI] [PubMed] [Google Scholar]
- 84.Dal Co A, van Vliet S, Ackermann M. 2019. Emergent microscale gradients give rise to metabolic cross-feeding and antibiotic tolerance in clonal bacterial populations. Philos Trans R Soc Lond B Biol Sci 374:20190080. 10.1098/rstb.2019.0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhang B, Deng H, Wang H, Yin R, Hallett PD, Griffiths BS, Daniell TJ. 2010. Does microbial habitat or community structure drive the functional stability of microbes to stresses following re-vegetation of a severely degraded soil? Soil Biol Biochem 42:850–859. 10.1016/j.soilbio.2010.02.004. [DOI] [Google Scholar]
- 86.Liao J, Ying Z, Hiebeler DE, Wang Y, Takada T, Nijs I. 2015. Species extinction thresholds in the face of spatially correlated periodic disturbance. Sci Rep 5:15455. 10.1038/srep15455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.König S, Worrich A, Centler F, Wick LY, Miltner A, Kästner M, Thullner M, Frank K, Banitz T. 2017. Modelling functional resilience of microbial ecosystems: analysis of governing processes. Environ Model Softw 89:31–39. 10.1016/j.envsoft.2016.11.025. [DOI] [Google Scholar]
- 88.König S, Worrich A, Banitz T, Harms H, Kästner M, Miltner A, Wick LY, Frank K, Thullner M, Centler F. 2018. Functional resistance to recurrent spatially heterogeneous disturbances is facilitated by increased activity of surviving bacteria in a virtual ecosystem. Front Microbiol 9:734. 10.3389/fmicb.2018.00734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.König S, Worrich A, Banitz T, Centler F, Harms H, Kästner M, Miltner A, Wick LY, Thullner M, Frank K. 2018. Spatiotemporal disturbance characteristics determine functional stability and collapse risk of simulated microbial ecosystems. Sci Rep 8:9488. 10.1038/s41598-018-27785-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.König S, Köhnke MC, Firle A-L, Banitz T, Centler F, Frank K, Thullner M. 2019. Disturbance size can be compensated for by spatial fragmentation in soil microbial ecosystems. Front Ecol Evol 7:290. 10.3389/fevo.2019.00290. [DOI] [Google Scholar]
- 91.Renslow RS, Lindemann SR, Song H-S. 2016. A generalized spatial measure for resilience of microbial systems. Front Microbiol 7:443. 10.3389/fmicb.2016.00443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Shade A, Read JS, Youngblut ND, Fierer N, Knight R, Kratz TK, Lottig NR, Roden EE, Stanley EH, Stombaugh J, Whitaker RJ, Wu CH, McMahon KD. 2012. Lake microbial communities are resilient after a whole-ecosystem disturbance. ISME J 6:2153–2167. 10.1038/ismej.2012.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tseng C-H, Chiang P-W, Shiah F-K, Chen Y-L, Liou J-R, Hsu T-C, Maheswararajah S, Saeed I, Halgamuge S, Tang S-L. 2013. Microbial and viral metagenomes of a subtropical freshwater reservoir subject to climatic disturbances. ISME J 7:2374–2386. 10.1038/ismej.2013.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Locey KJ, Lennon JT. 2019. A residence time theory for biodiversity. Am Nat 194:59–72. 10.1086/703456. [DOI] [PubMed] [Google Scholar]
- 95.Lindström ES, Langenheder S. 2012. Local and regional factors influencing bacterial community assembly: bacterial community assembly. Environ Microbiol Rep 4:1–9. 10.1111/j.1758-2229.2011.00257.x. [DOI] [PubMed] [Google Scholar]
- 96.Rillig MC, Antonovics J, Caruso T, Lehmann A, Powell JR, Veresoglou SD, Verbruggen E. 2015. Interchange of entire communities: microbial community coalescence. Trends Ecol Evol 30:470–476. 10.1016/j.tree.2015.06.004. [DOI] [PubMed] [Google Scholar]
- 97.Shade A, Caporaso JG, Handelsman J, Knight R, Fierer N. 2013. A meta-analysis of changes in bacterial and archaeal communities with time. ISME J 7:1493–1506. 10.1038/ismej.2013.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Rivest D, Paquette A, Shipley B, Reich PB, Messier C. 2015. Tree communities rapidly alter soil microbial resistance and resilience to drought. Funct Ecol 29:570–578. 10.1111/1365-2435.12364. [DOI] [Google Scholar]
- 99.von Rein I, Gessler A, Premke K, Keitel C, Ulrich A, Kayler ZE. 2016. Forest understory plant and soil microbial response to an experimentally induced drought and heat-pulse event: the importance of maintaining the continuum. Glob Chang Biol 22:2861–2874. 10.1111/gcb.13270. [DOI] [PubMed] [Google Scholar]
- 100.Fry EL, Manning P, Macdonald C, Hasegawa S, De Palma A, Power SA, Singh BK. 2016. Shifts in microbial communities do not explain the response of grassland ecosystem function to plant functional composition and rainfall change. Soil Biol Biochem 92:199–210. 10.1016/j.soilbio.2015.10.006. [DOI] [Google Scholar]
- 101.de Vries FT, Liiri ME, Bjørnlund L, Setälä HM, Christensen S, Bardgett RD. 2012. Legacy effects of drought on plant growth and the soil food web. Oecologia 170:821–833. 10.1007/s00442-012-2331-y. [DOI] [PubMed] [Google Scholar]
- 102.Bardgett RD, Manning P, Morriën E, De Vries FT. 2013. Hierarchical responses of plant-soil interactions to climate change: consequences for the global carbon cycle. J Ecol 101:334–343. 10.1111/1365-2745.12043. [DOI] [Google Scholar]
- 103.Simon M, Grossart H, Schweitzer B, Ploug H. 2002. Microbial ecology of organic aggregates in aquatic ecosystems. Aquat Microb Ecol 28:175–211. 10.3354/ame028175. [DOI] [Google Scholar]
- 104.Kaurin A, Mihelič R, Kastelec D, Grčman H, Bru D, Philippot L, Suhadolc M. 2018. Resilience of bacteria, archaea, fungi and N-cycling microbial guilds under plough and conservation tillage, to agricultural drought. Soil Biol Biochem 120:233–245. 10.1016/j.soilbio.2018.02.007. [DOI] [Google Scholar]
- 105.Grossart H-P. 1999. Interactions between marine bacteria and axenic diatoms (Cylindrotheca fusiformis, Nitzschia laevis, and Thalassiosira weissflogii) incubated under various conditions in the lab. Aquat Microb Ecol 19:1–11. 10.3354/ame019001. [DOI] [Google Scholar]
- 106.Bush T, Diao M, Allen RJ, Sinnige R, Muyzer G, Huisman J. 2017. Oxic-anoxic regime shifts mediated by feedbacks between biogeochemical processes and microbial community dynamics. Nat Commun 8:789. 10.1038/s41467-017-00912-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Röhl O, Graupner N, Peršoh D, Kemler M, Mittelbach M, Boenigk J, Begerow D. 2018. Flooding duration affects the structure of terrestrial and aquatic microbial eukaryotic communities. Microb Ecol 75:875–887. 10.1007/s00248-017-1085-9. [DOI] [PubMed] [Google Scholar]
- 108.Fukami T. 2001. Sequence effects of disturbance on community structure. Oikos 92:215–224. 10.1034/j.1600-0706.2001.920203.x. [DOI] [Google Scholar]
- 109.Darling ES, Côté IM. 2008. Quantifying the evidence for ecological synergies. Ecol Lett 11:1278–1286. 10.1111/j.1461-0248.2008.01243.x. [DOI] [PubMed] [Google Scholar]
- 110.Crain CM, Kroeker K, Halpern BS. 2008. Interactive and cumulative effects of multiple human stressors in marine systems. Ecol Lett 11:1304–1315. 10.1111/j.1461-0248.2008.01253.x. [DOI] [PubMed] [Google Scholar]
- 111.Paine RT, Tegner MJ, Johnson EA. 1998. Compounded perturbations yield ecological surprises. Ecosystems 1:535–545. 10.1007/s100219900049. [DOI] [Google Scholar]
- 112.Buma B. 2015. Disturbance interactions: characterization, prediction, and the potential for cascading effects. Ecosphere 6:1–15. 10.1890/ES15-00058.1. [DOI] [Google Scholar]
- 113.Bardgett RD, Caruso T. 2020. Soil microbial community responses to climate extremes: resistance, resilience and transitions to alternative states. Philos Trans R Soc Lond B Biol Sci 375:20190112. 10.1098/rstb.2019.0112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hawkes CV, Keitt TH. 2015. Resilience vs. historical contingency in microbial responses to environmental change. Ecol Lett 18:612–625. 10.1111/ele.12451. [DOI] [PubMed] [Google Scholar]
- 115.Connell JH. 1978. Diversity in tropical rain forests and coral reefs. Science 199:1302–1310. 10.1126/science.199.4335.1302. [DOI] [PubMed] [Google Scholar]
- 116.Odum EP. 1981. The effects of stress on the trajectory of ecological succession, p 43–47. In Barrett GW, Rosenberg R. (ed), Stress effects on natural ecosystems. John Wiley & Sons Ltd., Chichester, United Kingdom. [Google Scholar]
- 117.Bender EA, Case TJ, Gilpin ME. 1984. Perturbation experiments in community ecology: theory and practice. Ecology 65:1–13. 10.2307/1939452. [DOI] [Google Scholar]
- 118.Glasby TM, Underwood AJ. 1996. Sampling to differentiate between pulse and press perturbations. Environ Monit Assess 42:241–252. 10.1007/BF00414371. [DOI] [PubMed] [Google Scholar]
- 119.Eberlein C, Baumgarten T, Starke S, Heipieper HJ. 2018. Immediate response mechanisms of Gram-negative solvent-tolerant bacteria to cope with environmental stress: cis-trans isomerization of unsaturated fatty acids and outer membrane vesicle secretion. Appl Microbiol Biotechnol 102:2583–2593. 10.1007/s00253-018-8832-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Gonzalez A, Bell G. 2013. Evolutionary rescue and adaptation to abrupt environmental change depends upon the history of stress. Philos Trans R Soc Lond B Biol Sci 368:20120079. 10.1098/rstb.2012.0079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Schimel J, Balser TC, Wallenstein M. 2007. Microbial stress-response physiology and its implications for ecosystem function. Ecology 88:1386–1394. 10.1890/06-0219. [DOI] [PubMed] [Google Scholar]
- 122.Hartmann M, Brunner I, Hagedorn F, Bardgett RD, Stierli B, Herzog C, Chen X, Zingg A, Graf-Pannatier E, Rigling A, Frey B. 2017. A decade of irrigation transforms the soil microbiome of a semi-arid pine forest. Mol Ecol 26:1190–1206. 10.1111/mec.13995. [DOI] [PubMed] [Google Scholar]
- 123.Bouskill NJ, Wood TE, Baran R, Ye Z, Bowen BP, Lim H, Zhou J, Nostrand JDV, Nico P, Northen TR, Silver WL, Brodie EL. 2016. Belowground response to drought in a tropical forest soil. I. Changes in microbial functional potential and metabolism. Front Microbiol 7:525. 10.3389/fmicb.2016.00525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Bouskill NJ, Lim HC, Borglin S, Salve R, Wood TE, Silver WL, Brodie EL. 2013. Pre-exposure to drought increases the resistance of tropical forest soil bacterial communities to extended drought. ISME J 7:384–394. 10.1038/ismej.2012.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Evans SE, Wallenstein MD. 2012. Soil microbial community response to drying and rewetting stress: does historical precipitation regime matter? Biogeochemistry 109:101–116. 10.1007/s10533-011-9638-3. [DOI] [Google Scholar]
- 126.de Nijs EA, Hicks LC, Leizeaga A, Tietema A, Rousk J. 2019. Soil microbial moisture dependences and responses to drying-rewetting: the legacy of 18 years drought. Glob Chang Biol 25:1005–1015. 10.1111/gcb.14508. [DOI] [PubMed] [Google Scholar]
- 127.Jurburg SD, Nunes I, Brejnrod A, Jacquiod S, Priemé A, Sørensen SJ, Van Elsas JD, Salles JF. 2017. Legacy effects on the recovery of soil bacterial communities from extreme temperature perturbation. Front Microbiol 8:1832. 10.3389/fmicb.2017.01832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ho A, Lüke C, Reim A, Frenzel P. 2016. Resilience of (seed bank) aerobic methanotrophs and methanotrophic activity to desiccation and heat stress. Soil Biol Biochem 101:130–138. 10.1016/j.soilbio.2016.07.015. [DOI] [Google Scholar]
- 129.Fierer N, Schimel JP, Holden PA. 2003. Influence of drying-rewetting frequency on soil bacterial community structure. Microb Ecol 45:63–71. 10.1007/s00248-002-1007-2. [DOI] [PubMed] [Google Scholar]
- 130.Zhou X, Fornara D, Ikenaga M, Akagi I, Zhang R, Jia Z. 2016. The resilience of microbial community under drying and rewetting cycles of three forest soils. Front Microbiol 7:1101. 10.3389/fmicb.2016.01101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Phillips LA, Schefe CR, Fridman M, O'Halloran N, Armstrong RD, Mele PM. 2015. Organic nitrogen cycling microbial communities are abundant in a dry Australian agricultural soil. Soil Biol Biochem 86:201–211. 10.1016/j.soilbio.2015.04.004. [DOI] [Google Scholar]
- 132.de Vries FT, Liiri ME, Bjørnlund L, Bowker MA, Christensen S, Setälä HM, Bardgett RD. 2012. Land use alters the resistance and resilience of soil food webs to drought. Nat Clim Chang 2:276–280. 10.1038/nclimate1368. [DOI] [Google Scholar]
- 133.Kaisermann A, de Vries FT, Griffiths RI, Bardgett RD. 2017. Legacy effects of drought on plant-soil feedbacks and plant-plant interactions. New Phytol 215:1413–1424. 10.1111/nph.14661. [DOI] [PubMed] [Google Scholar]
- 134.Tlili A, Maréchal M, Bérard A, Volat B, Montuelle B. 2011. Enhanced co-tolerance and co-sensitivity from long-term metal exposures of heterotrophic and autotrophic components of fluvial biofilms. Sci Total Environ 409:4335–4343. 10.1016/j.scitotenv.2011.07.026. [DOI] [PubMed] [Google Scholar]
- 135.Philippot L, Cregut M, Chèneby D, Bressan M, Dequiet S, Martin-Laurent F, Ranjard L, Lemanceau P. 2008. Effect of primary mild stresses on resilience and resistance of the nitrate reducer community to a subsequent severe stress. FEMS Microbiol Lett 285:51–57. 10.1111/j.1574-6968.2008.01210.x. [DOI] [PubMed] [Google Scholar]
- 136.Renella G, Ortigoza ALR, Landi L, Nannipieri P. 2003. Additive effects of copper and zinc on cadmium toxicity on phosphatase activities and ATP content of soil as estimated by the ecological dose (ED50). Soil Biol Biochem 35:1203–1210. 10.1016/S0038-0717(03)00181-0. [DOI] [Google Scholar]
- 137.McTee M, Bullington L, Rillig MC, Ramsey PW. 2019. Do soil bacterial communities respond differently to abrupt or gradual additions of copper? FEMS Microbiol Ecol 95:fiy212. 10.1093/femsec/fiy212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Li J, Wang J-T, Hu H-W, Ma Y-B, Zhang L-M, He J-Z. 2016. Copper pollution decreases the resistance of soil microbial community to subsequent dry–rewetting disturbance. J Environ Sci (China) 39:155–164. 10.1016/j.jes.2015.10.009. [DOI] [PubMed] [Google Scholar]
- 139.Li J, Liu Y-R, Cui L-J, Hu H-W, Wang J-T, He J-Z. 2017. Copper pollution increases the resistance of soil archaeal community to changes in water regime. Microb Ecol 74:877–887. 10.1007/s00248-017-0992-0. [DOI] [PubMed] [Google Scholar]
- 140.Vinebrooke RD, Cottingham KL, Norberg J, Scheffer M, Dodson SI, Maberly SC, Sommer U. 2004. Impacts of multiple stressors on biodiversity and ecosystem functioning: the role of species co-tolerance. Oikos 104:451–457. 10.1111/j.0030-1299.2004.13255.x. [DOI] [Google Scholar]
- 141.Rillig MC, Rolff J, Tietjen B, Wehner J, Andrade-Linares DR. 2015. Community priming—effects of sequential stressors on microbial assemblages. FEMS Microbiol Ecol 91:fiv40. 10.1093/femsec/fiv040. [DOI] [PubMed] [Google Scholar]
- 142.Pesce S, Lambert A-S, Morin S, Foulquier A, Coquery M, Dabrin A. 2018. Experimental warming differentially influences the vulnerability of phototrophic and heterotrophic periphytic communities to copper toxicity. Front Microbiol 9:1424. 10.3389/fmicb.2018.01424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Sjöstedt J, Langenheder S, Kritzberg E, Karlsson CMG, Lindström ES. 2018. Repeated disturbances affect functional but not compositional resistance and resilience in an aquatic bacterioplankton community: effect of disturbances in bacterial communities. Environ Microbiol Rep 10:493–500. 10.1111/1758-2229.12656. [DOI] [PubMed] [Google Scholar]
- 144.Renes SE, Sjöstedt J, Fetzer I, Langenheder S. 2020. Disturbance history can increase functional stability in the face of both repeated disturbances of the same type and novel disturbances. Sci Rep 10:11333. 10.1038/s41598-020-68104-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Jurburg SD, Nunes I, Stegen JC, Le Roux X, Priemé A, Sørensen SJ, Salles JF. 2017. Autogenic succession and deterministic recovery following disturbance in soil bacterial communities. Sci Rep 7:45691. 10.1038/srep45691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Seneviratne M, Marschner P. 2019. Soil respiration and nutrient availability after short heating followed by rewetting differ between first and second heating and are influenced by the interval between heating events. Soil Biol Biochem 136:107537. 10.1016/j.soilbio.2019.107537. [DOI] [Google Scholar]
- 147.Ho A, van den Brink E, Reim A, Krause SMB, Bodelier PLE. 2016. Recurrence and frequency of disturbance have cumulative effect on methanotrophic activity, abundance, and community structure. Front Microbiol 6:1493. 10.3389/fmicb.2015.01493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Svoboda P, Lindström ES, Ahmed Osman O, Langenheder S. 2018. Dispersal timing determines the importance of priority effects in bacterial communities. ISME J 12:644–646. 10.1038/ismej.2017.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Geisen S, Briones MJI, Gan HJ, Behan-Pelletier VM, Friman VP, de Groot GA, Hannula SE, Lindo Z, Philippot L, Tiunov AV, Wall DH. 2019. A methodology to embrace soil biodiversity. Soil Biol Biochem 136:107536. 10.1016/j.soilbio.2019.107536. [DOI] [Google Scholar]
- 150.Tilman D, Wedin D, Knops J. 1996. Productivity and sustainability influenced by biodiversity in grassland ecosystems. Nature 379:718–720. 10.1038/379718a0. [DOI] [Google Scholar]
- 151.Graham EB, Krause S. 2020. Social media sows consensus in disturbance ecology. Nature 577:170–170. 10.1038/d41586-020-00006-7. [DOI] [PubMed] [Google Scholar]
- 152.Gelman A. 2014. Bayesian data analysis, 3rd ed. CRC Press, Boca Raton, FL. [Google Scholar]
- 153.Liu J, Meng Z, Liu X, Zhang X-H. 2019. Microbial assembly, interaction, functioning, activity and diversification: a review derived from community compositional data. Mar Life Sci Technol 1:112–128. 10.1007/s42995-019-00004-3. [DOI] [Google Scholar]
- 154.Fukami T. 2015. Historical contingency in community assembly: integrating niches. Annu Rev Ecol Evol Syst 46:1–23. 10.1146/annurev-ecolsys-110411-160340. [DOI] [Google Scholar]
- 155.Plante CJ. 2017. Defining disturbance for microbial ecology. Microb Ecol 74:259–263. 10.1007/s00248-017-0956-4. [DOI] [PubMed] [Google Scholar]
- 156.Pimm SL. 1984. The complexity and stability of ecosystems. Nature 307:321–326. 10.1038/307321a0. [DOI] [Google Scholar]
- 157.Strona G, Veech JA. 2015. A new measure of ecological network structure based on node overlap and segregation. Methods Ecol Evol 6:907–915. 10.1111/2041-210X.12395. [DOI] [Google Scholar]
- 158.Keddy PA. 1992. Assembly and response rules: two goals for predictive community ecology. J Veg Sci 3:157–164. 10.2307/3235676. [DOI] [Google Scholar]
- 159.Jax K. 2005. Function and “functioning” in ecology: what does it mean? Oikos 111:641–648. 10.1111/j.1600-0706.2005.13851.x. [DOI] [Google Scholar]
- 160.Hubbell SP. 2005. Neutral theory in community ecology and the hypothesis of functional equivalence. Funct Ecol 19:166–172. 10.1111/j.0269-8463.2005.00965.x. [DOI] [Google Scholar]
- 161.Manning PK. 2017. Ecosystem multifunctionality. Ecology. Oxford University Press, Oxford, United Kingdom. [Google Scholar]
- 162.Johnson NC, Graham JH, Smith FA. 1997. Functioning of mycorrhizal associations along the mutualism-parasitism continuum. New Phytol 135:575–585. 10.1046/j.1469-8137.1997.00729.x. [DOI] [Google Scholar]
- 163.Bais HP, Weir TL, Perry LG, Gilroy S, Vivanco JM. 2006. The role of root exudates in rhizosphere interactions with plants and other organisms. Annu Rev Plant Biol 57:233–266. 10.1146/annurev.arplant.57.032905.105159. [DOI] [PubMed] [Google Scholar]
- 164.Finegan B. 1984. Forest succession. Nature 312:109–114. 10.1038/312109a0. [DOI] [Google Scholar]
- 165.Cook WM, Yao J, Foster BL, Holt RD, Patrick LB. 2005. Secondary succession in an experimentally fragmented landscape: community patterns across space and time. Ecology 86:1267–1279. 10.1890/04-0320. [DOI] [Google Scholar]