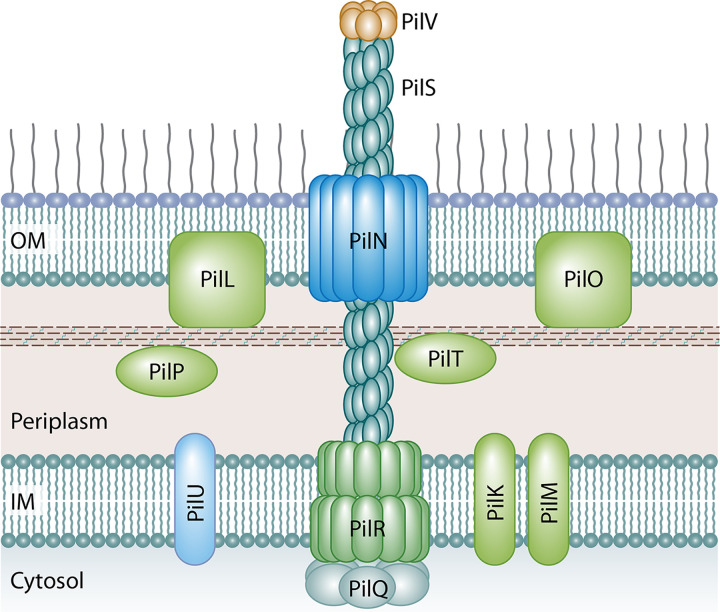
FIG 6.
Predicted structure of the T4P of IncI1 plasmids based on homologs from the better-characterized TCP and BFG pili. PilS forms the major prepilin polymer complex that extends from the cell to form the T4P and is capped by PilV subunits that make up the minor prepilin multimer that interacts with specific oligosaccharide in LPS on a recipient cell. Other structural elements include PilR, which is the integral inner membrane spanning protein, and PilN, which forms the outer membrane secretin through which the PilS polymer extends. PilT is predicted to be a lytic transglycosidase that may function to create a pore through the peptidoglycan layer to allow elongation of the pilus structure. PilR proteins are predicted to be platform proteins that transfer energy from the system ATPases, such as PilQ, to the T4P structure.