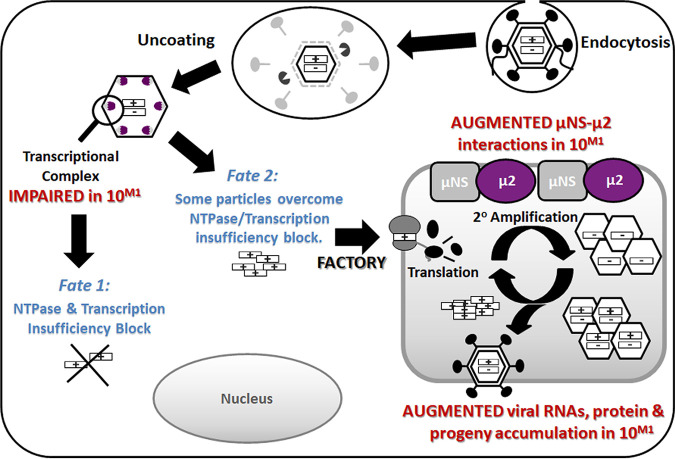
FIG 9.
Final model. Reovirus variant T3v10M1 has a single amino acid alteration, A612V, in the previously uncharacterized C-terminal loop of μ2. The alteration does not change the levels of cell attachment protein σ1, and therefore it binds and uncoats in a similar manner to that of T3wt. As a polymerase cofactor and a major part of the transcriptional complex (λ3-μ2), the single-residue replacement impairs NTPase activities and RNA synthesis and causes ∼80% of virions to fail to initiate productive infection (producing detectable viral proteins) (fate 1). However, for the T3v10M1 virus particles that are capable of overcoming the transcriptional limitations, T3v10M1 μ2 produces increased associations with μNS and promotes progeny synthesis (fate 2). Overall, the A612V mutation in μ2 produces a negative impact (fate 1) and a positive impact (fate 2) on distinct stages of virus replication, but the net sum of these fates is beneficial, as indicated by increased plaque size and burst size in virus growth curves.