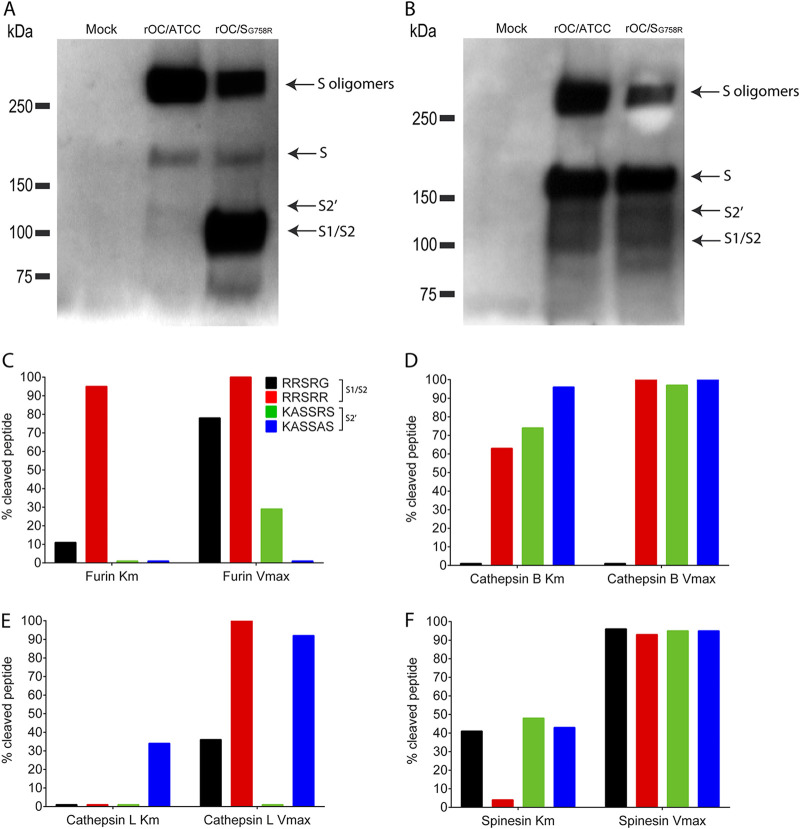
FIG 3.
Cathepsins B and L and TMPRSS5/spinesin are potential players in S glycoprotein cleavage. Differentiated LA-N-5 cells were infected with rOC/ATCC or rOC/SG758R at an MOI of 0.2. Proteins in association with cells or in supernatant were extracted at 48 hpi, and Western blot analysis of cell culture supernatant (A) or whole-cell lysates (B) (10 μg of proteins loaded in all wells as measured by BCA) revealed the presence of oligomers of S proteins (250 kDa), the uncleaved monomer form of the S glycoprotein (180 kDa), a cleaved form at about 100 kDa (S1/S2), and presumably the second cleaved site at about 120 kDa (S2′). Synthetic peptides represent all virus S protein at the S1/S2 site, RRSRG (rOC/ATCC; black) and RRSRR (rOC/SG758R; red), or at S2′ site, KASSRS (rOC/ATCC; green) and KASSAS (rOC/SR903A; blue). The different peptides were incubated with furin (C), cathepsin B (D) or L (E), or TMPRSS5/spinesin (F) for 30 min, and percent cleaved peptide was measured as described in Materials and Methods. Cleavage at Km is a representation of an early step of viral infection (small amount of peptide) and Vmax a late step of viral infection (large amount of peptide).