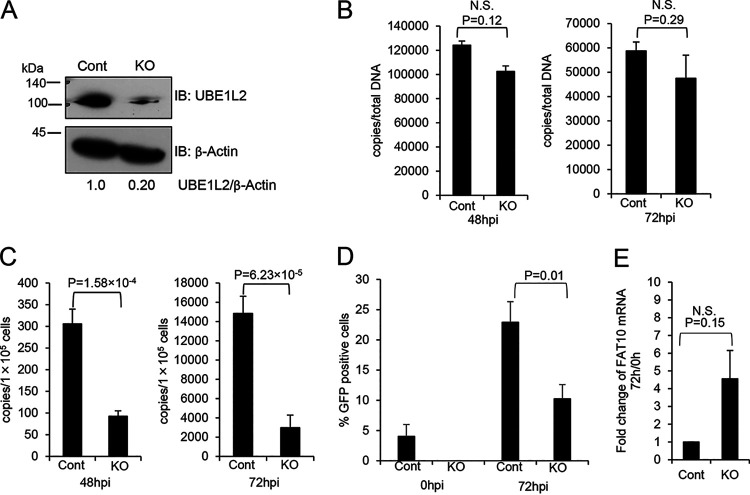
FIG 3.
KO of UBE1L2 in host cells reduces KSHV production but not KSHV DNA synthesis. (A) CRISPR/Cas9-mediated KO of UBE1L2 in iSLK-rKSHV.219 cells. iSLK-rKSHV.219 cells were transfected with the UBE1L2 CRISPR/Cas9 KO plasmid (KO) or control CRISPR/Cas9 plasmid (Cont) and cultured for 4 days. The UBE1L2 KO was confirmed by immunoblotting (IB) with anti-UBE1L2 antibody. The UBE1L2 and β-actin band intensities were calculated using ImageJ software, and the value of UBE1L2/β-actin in the control is presented as 1.0. (B to E) Effects of UBE1L2 KO on KSHV DNA synthesis (B), virus production (C), infectious virus production (D), and FAT10 mRNA expression (E) in iSLK-rKSHV.219 cells. In order to induce lytic replication (viral DNA synthesis and progeny virus production), the control (Cont) and UBE1L2 KO (KO) cells were treated with Dox and NaB. (B) At 48 or 72 h postinduction (hpi), the intracellular viral DNA synthesis levels were evaluated by real-time qPCR as described in Materials and Methods. (C) At 48 or 72 hpi, the extracellular KSHV production was quantitated by real-time qPCR of viral DNA derived from mature virions in the culture supernatant. (D) At 0 or 72 hpi, the culture supernatants were collected and inoculated with 293T cells for infection. At 72 h postinfection, GFP-positive cells were counted by fluorescence microscopy. (E) The expression levels of FAT10 mRNA were not affected by UBE1L2 KO. At 0 or 72 h posttreatment, cells were collected, and total RNA was subjected to real-time RT-qPCR. The FAT10 mRNA levels were normalized toward the GAPDH mRNA levels. The mRNA expression levels shown in the graphs were obtained by dividing the mRNA expression values from 72 h by those from 0 h to monitor the rate of increase. The two-tailed Student’s t test was used to indicate between-group differences. The P values are shown in each graph. N.S., not significant.