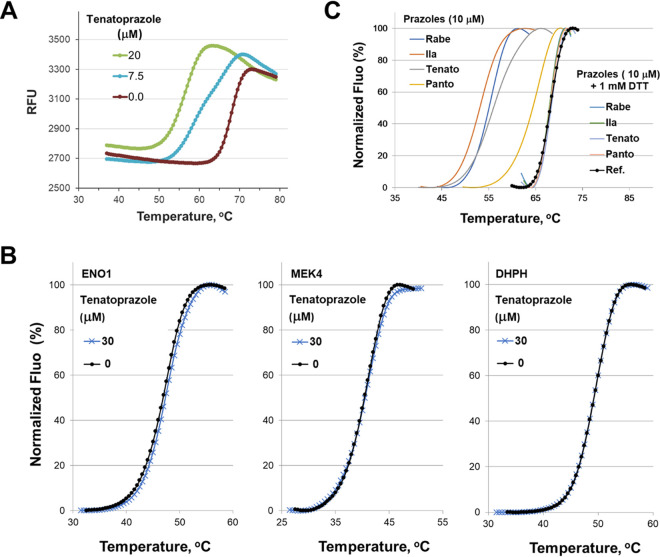
FIG 1.
Thermal shift data of Tsg101 by the lead compound tenatoprazole (N16). (A) The compound caused a dose-dependent shift in the Tm for Tsg101-UEV, indicating binding to the key domain of Tsg101, as described in Materials and Methods. RFU, relative fluorescence units. (B) DHPH, ENO1, and MEK4 do not cause a thermal shift with Tsg101. Thermal shift data of three human proteins not related to Tsg101 by the lead compound tenatoprazole are shown. The effect of the prazole compound on the thermal stability of these three proteins is negligible, indicating that the dramatic modulation of the thermal transition of Tsg101 by the prazoles is due to a specific interaction. (C) The addition of DTT abolishes the Tm shift in the FTS assay, consistent with prazole compounds forming a disulfide bond to Tsg101. Rabe, rabeprazole; Ila, ilaprazole; Tenato, tenatoprazole; Panto, pantoprazole; Ref., reference; Fluo, fluorescence.