Abstract
Summary We report a case of pituicytoma, a rare primary tumor of the neurohypophysis. A 64-year-old man presented with progressive visual complaints, bitemporal hemianopsia, and headache. Imaging studies revealed distinctive features of a mass lesion that thickened the pituitary stalk with a bilobed protrusion extending into the hypothalamus. Angiography demonstrated tumor vascular supply from the superior hypophyseal arteries representing the diencephalic branches of the internal carotid arteries. We discuss the imaging and pathology of this unusual tumor.
Pituicytomas are very rare primary tumors of the adult neurohypophysis, and only a few case reports of true pituicytomas exist in the literature.1–9 Pituicytes are glial cells of the neurohypophysis that support the large axons of vasopressin and oxytocin-producing hypothalamic neurons. Pituicytomas are considered low-grade astrocytomas of the neurohypophysis, distinct from intracranial granular cell tumors (GCT) and pilocytic astrocytomas. They are histologically benign, but their hypervascular nature makes surgical resection difficult.2 Local recurrence after subtotal resection is common.1 The imaging characteristics of pituicytomas are nonspecific, but MR imaging and carotid angiography are vital for surgical planning and can reveal detailed diagnostic information. Ours is the first presentation of specific angiographic findings of a pituicytoma. Although not diagnostic, the appearance of enlarged superior hypophyseal and hypothalamic vessels supplying a thickened pituitary stalk localized the tumor to the neurohypophysis.
Case Report
A 64-year-old man was referred to our institution for evaluation of a suprasellar mass, presenting with bitemporal hemianopsia. The referral diagnosis was pituitary macroadenoma. The patient described a 14-year history of headaches, which had recently increased in severity. During the past 2 years, the patient developed slurred speech, decreased balance, dizziness, and short-term memory deficits. He denied nausea and vomiting, as well as any recent illness. The patient’s medical history was significant for bipolar disorder and depression, which had been treated during 18 years of psychiatric care. He reported a 30-year history of dizziness, headaches, and double vision. In addition, 30 years ago, the patient underwent orchiectomy for testicular cancer, received radiation treatment, and has received testosterone replacement since that time.
Findings of an ophthalmology examination revealed 20/40 vision in the right eye and 20/30 in the left eye. Visual field testing showed bitemporal hemianopsia consistent with chiasmatic compression.
Findings of preoperative neuroendocrine studies were normal except for a slightly elevated prolactin level of 46.8 ng/mL, thought to be secondary to stalk disinhibition effect. All other aspects of the physical examination were normal.
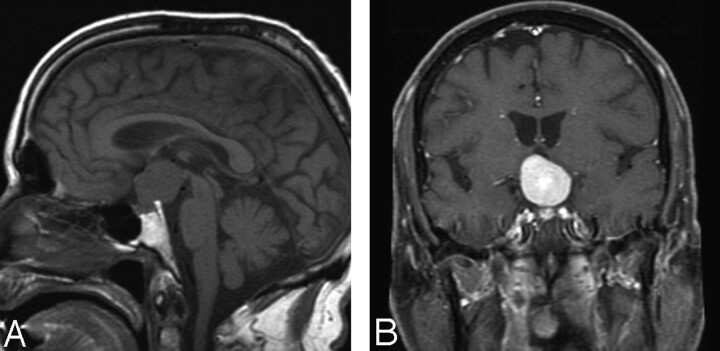
MR imaging revealed a 2.9 × 2.6 × 2.5 homogeneously enhancing mass in the suprasellar cistern, extending into the tentorial incisura and upper posterior fossa above the upper one third of the clivus (Fig 1 A, -B). It appeared to be centered on the posterior clinoid and the diaphragma sella. It was not clear whether the tumor had a dural attachment at the diaphragma sella. The mass had intermediate signal intensity on T1-weighted images, intermediate-to-slightly-increased signal intensity on T2-weighted images, and marked homogeneous enhancement with contrast administration. CT images showed no calcifications, necrosis, bone destruction, or hyperostosis.
Fig 1.
A, Sagittal MR image shows a rounded mass in the suprasellar region, extending to the third ventricle. B, Coronal enhanced scan shows intense enhancement of the mass lesion, which appears to originate in the suprasellar region with fairly well-defined margins. The pituitary stalk, however, is not seen as a structure separate from the mass.
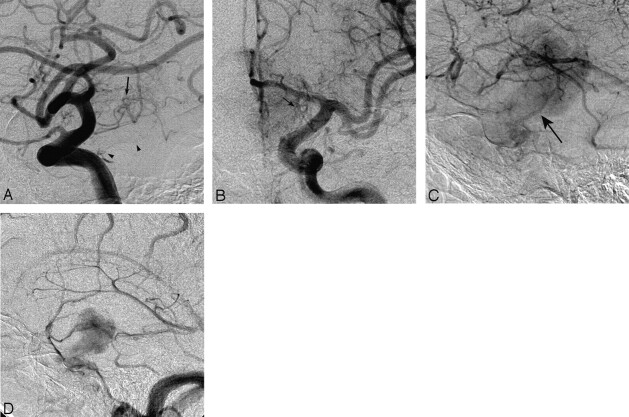
The predominantly suprasellar location of the mass led to uncertainty about the referral diagnosis. An angiogram was obtained to assess for the possibility of meningioma or hemangiopericytoma. Findings of selective external carotid artery (ECA) injections were negative, with no ECA dural feeders. A selective internal carotid angiogram demonstrated capillary blush consistent with a hypervascular tumor, which initially suggested a meningioma or hemangiopericytoma (Fig 2 A–D). On the selective right internal carotid artery angiogram, the tumor was shown to exert some mass effect on the right supraclinoid internal carotid artery, displacing it anteriorly and medially. There was no evidence of encasement or arteriovenous shunt surgery. On the late capillary and venous phase, there was some evidence of mild retention of the capillary filling of the tumor. The selective left internal carotid artery angiogram showed mass effect on the supraclinoid internal carotid artery and A1 segment of the left anterior cerebral artery. An MR angiogram showed upward displacement of both anterior cerebral artery A1 segments.
Fig 2.
Selective bilateral internal carotid artery (ICA) angiograms. A, Early arterial lateral, (B) late arterial anteroposterior (AP), and (C) venous lateral magnified views of the left ICA injection. There are numerous vascular pedicles arising from the supraclinoid portion of the ICA that represent the various inferior and superior hypophyseal branches (arrows, A and B) that supply the neurohypophysis and hypothalamus. The meningohypophyseal trunk, which supplies the inferior hypophyseal artery as well as the dorsal meningeal artery, is also visible, arising from the posterior genu of the cavernous ICA (arrowheads). During the venous phase (C), the tumor stain is apparent, extending from the suprasellar region upwards in a “dumbbell” or “mushroom” pattern. There is a prominent portal vein draining into the dural venous sinus (arrow). D, Late venous phase of the lateral injection of the right ICA. The shape of the tumor stain is well seen with a caudad extension along the enlarged pituitary stalk and cephalad extension into the hypothalamus. The delayed tumor stain and prominence of the meningohypophyseal trunk at first glance suggest a meningioma of the diaphragma sella and suprasellar region.
A skull base microsurgical approach consisting of a right frontal-temporal craniotomy with orbitozygomatic osteotomy was chosen to minimize brain retraction and facilitate a low inferior-to-superior line of direct visualization for dissecting the superior pole of the tumor, which elevated the floor of the third ventricle. Surgical exposure revealed a tumor with a pinkish-rose color and smooth surface. The tumor was found to be extremely vascular, and any physical manipulation of the capsule or substance of the tumor led to significant bleeding. Multiple blood vessels feeding the tumor arose from both internal carotid arteries, the right posterior communicating artery, both P1 segments of the posterior cerebral artery, the floor of the hypothalamus, and the inferior aspect of the chiasm. Although there was no direct tumor attachment to the dura, obvious feeding branches from the dura overlying the upper clivus, posterior clinoids, and diaphragma sella were present bilaterally. The tumor mass abutted the diaphragma sella and appeared to arise predominantly from the posterior aspect of the pituitary stalk, with extension up into the hypothalamus. Near the floor of the third ventricle, the pituitary stalk became diaphanous, and it was impossible to distinguish stalk from tumor. The stalk was sacrificed at the level of the hypothalamus to completely remove the tumor.
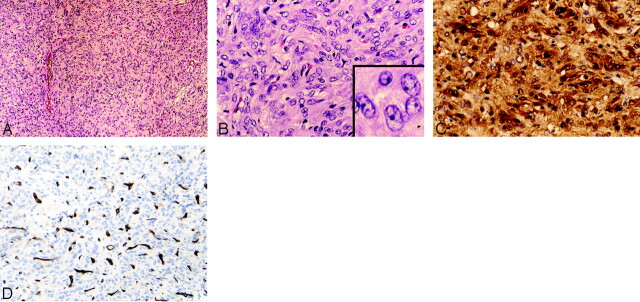
Microscopic examination of the tumor specimen showed a benign spindle cell neoplasm with a rich capillary network. The tumor had a storiform appearance and lacked collagen bands (Fig 3A). The spindle cells had abundant cytoplasm and characteristic nuclei, with large open chromatin and prominent small single nucleoli (Fig 3B). There was little-to-no mitotic activity. On histochemical stain, little-to-no reticulin was present around individual tumor cells. An immunoperoxidase panel was performed, including glial fibrillary acidic protein (GFAP), S-100, epithelial membrane antigen (EMA), CD31, CD34, HMB 45, Melan-A, neurofilament (NFP), synaptophysin, and Ki-67. There was strong diffuse positive staining for S-100 (Fig 3C). Staining for both epithelial membrane antigen (EMA) and GFAP was focal and weak. CD31 and CD34 highlighted the rich vascularity of the tumor, but tumor cells were negative for the endothelial markers (Fig 3D). Melanoma markers HMB 45 and Melan-A were negative, as were the neuronal markers synaptophysin and NFP. The proliferation index was low to moderate, with up to 5% of tumor nuclei positive for Ki-67.
Fig 3.
Tumor histopathology.
A, The solid and cellular neoplasm is composed of rounded-to-spindled cells growing in a storiform pattern. No necrosis, nuclear palisading (Schwannoma), whorls (meningioma), or collagen bands (solitary fibrous tumor) are detected. Occasional large-caliber vessels are present, but the rich capillary network of the tumor is difficult to discern at low power (H&E, original magnification ×10).
B, The tumor cells have abundant eosinophilic cytoplasm that lacks granularity. Tumor cell nuclei are large and have open chromatin, and many have a small distinct nucleolus. The rich capillary network is now visible. No mitotic figures, Rosenthal fibers, or eosinophilic granular bodies (pilocytic astrocytoma) are seen (H&E, original magnification ×20).
C, The tumor shows strong and diffuse staining for S-100 in both the cytoplasm and nuclei of individual tumor cells (S-100, H&E, original magnification ×20, ×40).
D, The endothelial cell marker CD34 highlights the rich vascular/capillary network of the tumor, whereas tumor cells are negative for this marker (CD34 stain, original magnification ×20).
Discussion
Very few cases of true pituicytomas have been described in the literature. Until 2000, when Brat et al1 described a series of 9 cases and clarified the definition of pituicytoma, intracranial GCTs (also called “choristomas” or “granular cell myoblastomas”) and pilocytic astrocytomas of the neurohypophysis were often included in the term “pituicytoma.” The classification of this tumor has been difficult because the histogenesis of pituicytomas and intracranial GCTs is unknown. Both are thought to originate from pituicytes, but they are distinct tumor types. Takai et al10 described 5 classes of pituicyte on the basis of ultrastructural features, which they believed to be different functional forms of the same cell line. They hypothesized that the distinct tumor types may arise from pituicytes in various stages of differentiation. Other authors have speculated that pituicytomas may arise from folliculostellate cells of the adenohypophysis, but no significant evidence substantiates this theory.2,6
Pituicytes are glial cells occupying perivascular zones of the neurohypophysis that regulate hypothalamic hormone release. Vasopressin and oxytocin are synthesized in the magnocellular nuclei and transported down axons that terminate in the neurohypophysis. In the basal state, these specialized astrocytes engulf and interpose themselves between the neurosecretory processes to physically block hormone release. When activated, they undergo dramatic morphologic change, retracting from axon terminals and the basal lamina to allow hormone release into the perivascular spaces to reach the fenestrated capillaries.11 Adenosine triphosphate, co-released with the hormones from axon terminals, is quickly broken down into adenosine by neurohypophyseal enzymes. Studies have shown that adenosine induces pituicyte stellation, the transformation of flat fusiform pituicytes into retracted round bodies with complex arborization, and vasopressin reverses the process. Rosso et al12 believe that this implies a negative feedback mechanism between the neurons and glia of the neurohypophysis.
Pituicytomas are composed of spindle- or stellate-shaped cells with round-oval-to-elongated irregular nuclei and syncytial fibrillary cytoplasm. These tumors are characterized by a storiform architecture and a rich capillary network. The arrangement of spindle cells around blood vessels is similar to the normal neurohypophyseal architecture, an important characteristic for identifying these tumors.7 The distinct antigenic profile includes diffusely positive staining for S-100 and vimentin, as well as variable staining for GFAP and EMA; other neural markers, such as synaptophysin, chromogranin A, and neurofilament are typically negative. Ki-67 immunostaining has been reported as low, up to 2%.1 In our patient, the proliferation index was low to moderate, occasionally up to 5%. In contrast, intracranial GCTs are formed of sheets of large polyhedral cells, with abundant eosinophilic cytoplasm filled with periodic acid-Schiff–positive granules.1 They have only focal spindled areas, and immunoreactivity for GFAP is uncommon.3 Extracranial GCTs originate from Schwann cells, and the histogenesis of intracranial GCTs is still debated. Pituicytomas are considered low-grade astrocytomas but are distinct from pilocytic astrocytomas. The latter are identified by a biphasic pattern of compact spindle cells with loose microcystic components, heavily fibrillated areas that stain intensely for GFAP, Rosenthal fibers, and granular cell bodies, all of which are absent in pituicytomas.1
Pituicytomas are benign, causing symptoms when they become large enough to displace and compress surrounding structures. Although previously described as noninfiltrating, this characterization is called into question by this case in which the pituitary stalk was found to be diffusely infiltrated at its superior aspect. Clinical presentation can include bitemporal visual field defects and headache, when the tumor is located in the sella, and psychiatric symptoms, when the tumor is suprasellar, compressing the hypothalamus. Treatment is surgical. Fractionated radiation therapy has been recommended for subtotal resection,2 but there are little outcome data to suggest differential benefit to radiation therapy in the setting of gross total resection. Recurrence after subtotal resection is common, occurring in 4 of the 7 cases reported in the literature for which a follow-up of greater than 6 months was available.1–4,9 With total resection, the prognosis is good; none of the patients who received total resection had recurrences at reported follow-up. The highly vascular nature of the tumor and its potential for infiltration can make total resection difficult. Subtotal resection due to extensive bleeding occurred in both of the cases reported by Ulm et al.2 In our patient, bleeding began as soon as the capsule was manipulated and continued throughout the procedure, with a total blood loss of 500 mL.
Imaging features are nonspecific, but MR images combined with angiograms are critical for surgical planning and can offer valuable clues in the diagnosis of this rare tumor. In this patient, the mass demonstrated MR imaging features similar to those in the few other cases reported in the literature.1 It was isointense on T1-weighted images and enhanced homogeneously with gadolinium administration. There was neither necrosis, calcification, nor flow voids. The delayed blush of the tumor on angiography demonstrated that it was extremely vascular. Prominent arterial feeding from the superior hypophyseal arteries, which supply both the diaphragma sella and the pituitary stalk, and the appearance of a thickened stalk suggested the infundibular origin of the tumor (Fig 4). Distinguishing this vascular pattern from diaphragma sella meningioma or posterior clinoid meningioma can be problematic, but the absence of external carotid artery dural feeders favors pituicytoma. To our knowledge, this is the first case in which a symptomatic pituicytoma has been characterized by MR imaging, MR angiography, and conventional angiography, as well as by light microscopy and immunohistochemical analysis.
Fig 4.
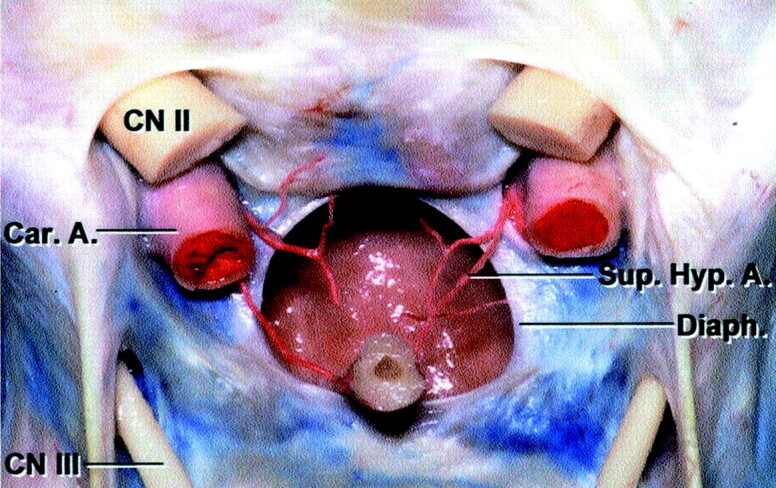
Color anatomic dissection of the sellar region. Frontal view of the optic chiasm and dissected diaphragma sella. The superior hypophyseal arteries are seen to branch into the pituitary stalk (Pit. Stalk) and extend cephalad toward the optic chiasm. (Reprinted with permission from Rhoton AL Jr. The sellar region. Neurosurgery 2002;51(suppl 1):335–74, [8.1])
As in our case, most pituicytomas are originally thought to be meningiomas. Although GCT is the most common of the neurohypophysis, pituicytoma should be added to the differential diagnosis of a sellar or suprasellar homogeneously enhancing solid mass, with numerous feeding vessels depicted by angiography. Recognition of this possibility will alert neurosurgeons to the risk of significant bleeding from this highly vascular tumor.
References
- 1.Brat DJ, Scheithauer BW, Staugaitis SM, et al. Pituicytoma: a distinctive low-grade glioma of the neurohypophysis. Am J Surg Pathol 2000;24:362–68 [DOI] [PubMed] [Google Scholar]
- 2.Ulm AJ, Yachnis AT, Brat DJ, et al. Pituicytoma: report of two cases and clues regarding histogenesis. Neurosurgery 2004;54:753–57 [DOI] [PubMed] [Google Scholar]
- 3.Kowalski RJ, Prayson RA, Mayberg MR. Pituicytoma. Ann Diagn Pathol 2004;8:290–94 [DOI] [PubMed] [Google Scholar]
- 4.Katsuta T, Inoue T, Nakagaki H, et al. Distinctions between pituicytoma and ordinary pilocytic astrocytoma: case report. J Neurosurg 2003;98:404–06 [DOI] [PubMed] [Google Scholar]
- 5.Uesaka T, Miyazono M, Nishio S, et al. Astrocytoma of the pituitary gland (pituicytoma): case report. Neuroradiology 2002;44:123–25 [DOI] [PubMed] [Google Scholar]
- 6.Cenacchi G, Giovenali P, Castrioto C, et al. Pituicytoma: ultrastructural evidence of a possible origin from folliculo-stellate cells of the adenohypophysis. Ultrastruct Pathol 2001;25:309–12 [DOI] [PubMed] [Google Scholar]
- 7.Schultz AB, Brat DJ, Oyesiku NM, et al. Intrasellar pituicytoma in a patient with other endocrine neoplasms. Arch Path Lab Med 2001;125:527–30 [DOI] [PubMed] [Google Scholar]
- 8.Hurley T, D’Angelo C, Clasen R, et al. Magnetic resonance imaging and pathological analysis of a pituicytoma: case report. Neurosurgery 1994;35:314–17 [DOI] [PubMed] [Google Scholar]
- 9.Figarella-Branger D, Dufour H, Fernandez C, et al. Pituicytomas, a mis-diagnosed benign tumor of the neurohypophysis: report of three cases. Acta neuropathologica 2002;104:313–19 [DOI] [PubMed] [Google Scholar]
- 10.Takei Y, Seyama S, Pearl GS, et al. Ultrastructural study of the human neurohypophysis. II. Cellular elements of neural parenchyma, the pituicytes. Cell Tissue Res 1980;205:273–87 [DOI] [PubMed] [Google Scholar]
- 11.Hatton GI. Pituicytes, glia and control of terminal secretion. J Exp Biol 1988;139:67–79 [DOI] [PubMed] [Google Scholar]
- 12.Rosso L, Peteri-Brunback B, Mienville JM. Putative physiological significance of vasopressin V1a receptor activation in rat pituicytes. J Neuroendocrinol 2004;16:313–18 [DOI] [PubMed] [Google Scholar]