Table 2.
Prespecified subgroup analysis of progression-free survival.
| Subgroup | Clarithromycin Group | Control Group | Clarithromycin Group | Control Group | Hazard ratio (95% CI) | |
|---|---|---|---|---|---|---|
| no. of progression events or deaths/total no. | median progression-free survival (mo) | |||||
| Sex | ||||||
| Male | 37/71 | 32/64 | 23 | 26 | 1.24 (0.77–1.98) | |
| Female | 33/71 | 29/79 | 35 | 39 | 1.38 (0.84–2.27) | |
| Age | ||||||
| <75yr | 26/64 | 26/59 | NR | 33 | 1.02 (0.59–1.75) | |
| ≥75yr | 44/78 | 35/84 | 19 | 28 | 1.56 (1.00–2.42) | |
| ISS | ||||||
| I | 10/36 | 11/33 | NR | 33 | 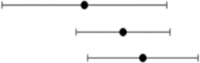 |
0.90 (0.38–2.13) |
| II | 32/52 | 31/59 | 15 | 25 | 1.35 (0.82–2.20) | |
| III | 28/54 | 19/51 | 17 | NR | 1.67 (0.94–2.98) | |
| Baseline creatinine clearance | ||||||
| >60 ml/min | 63/131 | 56/132 | 23 | 29 | 1.29 (0.90–1.84) | |
| ≤60 ml/min | 7/11 | 5/11 | 9 | NR | 1.65 (0.52–5.20) | |
| Type of MM | ||||||
| IgG | 29/74 | 32/84 | 35 | 39 | 1.06 (0.64–1.74) | |
| Non-IgG | 41/68 | 29/59 | 16 | 28 | 1.50 (0.93–2.40) | |
| Cytogenetic profile | ||||||
| High risk | 17/25 | 9/22 | 12 | NR |  |
2.16 (0.96–4.86) |
| Standard risk | 48/106 | 43/108 | 27 | 33 | 1.22 (0.81–1.84) | |
| ECOG score | ||||||
| 0 | 11/32 | 18/41 | 39 | 33 |  |
0.60 (0.28–1.27) |
| 1 | 30/64 | 22/61 | 26 | NR | 1.36 (0.79–2.36) | |
| ≥2 | 29/46 | 21/41 | 11 | 29 | 1.99 (1.13–3.51) | |
| R-ISS | ||||||
| I | 3/15 | 6/17 | NR | 29 | 0.68 (0.17–2.74) | |
| II | 42/79 | 36/83 | 23 | 29 | 1.21 (0.77–1.88) | |
| III | 16/23 | 5/18 | 7 | NR | 3.89 (1.41–10.7) | |
Shown are the results of an analysis of progression-free survival in prespecified subgroups of the intention-to-treat population that were defined according to baseline characteristics. The experimental group was treated with clarithromycin, lenalidomide and dexamethasone; the control group received lenalidomide and dexamethasone. The International Staging System (ISS) has three stages defined as follows: I (serum β2-microglobulin level < 3.5 mg/L and albumin level ≥ 3.5 g/dL; stage II, neither stage I nor III; and stage III serum β2-microglobulin level ≥ 5.5 mg/L. A high-risk cytogenetic profile was defined by a funding of t(4;14), t(14;16), or del17p on fluorescence in situ hybridization testing. The R-ISS is obtained on the basis of the combination of ISS, chromosomal abnormalities (CA) detected by interphase fluorescent in situ hybridization after CD138 plasma cell purification and serum lactate dehydrogenase (LDH). R-ISS I includes ISS stage I, no high-risk CA [del(17p) and/or t(4;14) and/or t(14;16)], and normal LDH level (less than the upper limit of normal range); R-ISS III including ISS stage III and high-risk CA or high LDH level; and R-ISS II, including all other possible combinations. Eastern Cooperative Oncology Group (ECOG) performance status is scored on a scale from 0 to 5, with higher scores indicating increasing disability.
