Fig. 5. Benefits of Adjuvant Systems over Alum for vaccine antibody responses.
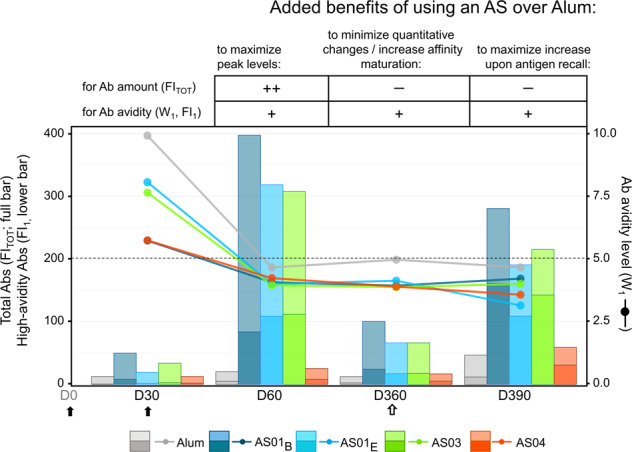
Schematic presentation of the benefits of using an Adjuvant System (AS) over Alum to formulate HBsAg vaccines is presented for the different aspects of the antibody (Ab) response. The immunization schedule comprised two primary vaccinations administered at day (D)0 and D30 (solid arrows) and a non-adjuvanted antigen recall (open arrow) administered at D360. The table summarizes the expected added benefits of the adjuvant choice (an AS or Alum) for enhancement of quantitative and qualitative aspects of the response at different timepoints post-vaccination or after antigen recall; ‘++’ and ‘+’ denote significant and moderate-to-slight enhancement, respectively, and ‘–’ denotes no clear benefit, i.e., similar proportional decline/enhancement for AS and Alum. The graph shows the abundance of HBsAg-specific Abs (total fluorescence intensity [FITOT]; full bars), the relative abundance of high-avidity specific Abs (fluorescence intensity of the first component Abs [FI1]; the bottom part of bars), and Ab avidity levels (W1; lines/symbols). Bars, lines, and symbols are color-coded according to the adjuvant groups presented below the graph. The horizontal dotted line represents the threshold for high avidity, with high-avidity profiles defined as W1 < 5.
