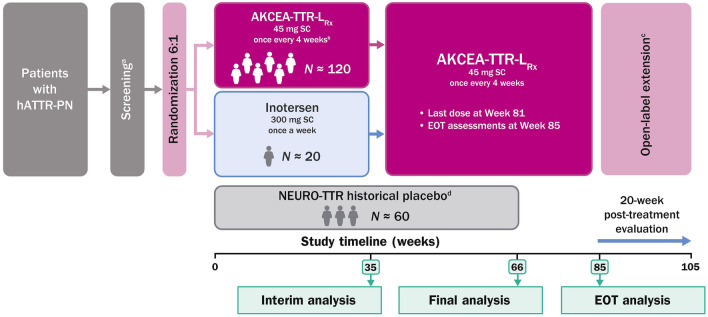
Fig. 2.
NEURO-TTRansform study design. EOT end of treatment, hATTR-PN hereditary transthyretin-mediated amyloid polyneuropathy, SC subcutaneously. aThe screening period is ≤ 6 weeks (or ≤ 10 weeks if genetic testing is required). bConcomitant therapy with tafamidis or off-label use of diflunisal is not allowed. Doxycycline use for the indication of infection (< 15 days) is allowed. cPatients not participating in the open-label extension will enter a 20-week post-treatment evaluation after completing EOT assessments. dPlacebo arm of the NEURO-TTR study (NCT01737398)