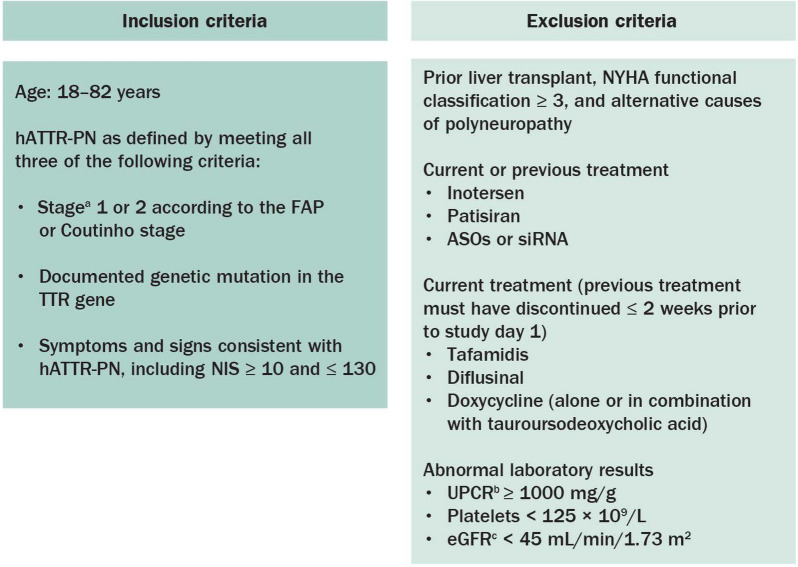
Fig. 4.
Key inclusion and exclusion criteria for the NEURO-TTRansform study. ASO antisense oligonucleotide, eGFR estimated glomerular filtration rate, FAP familial amyloid polyneuropathy, hATTR-PN hereditary transthyretin-mediated amyloid polyneuropathy, NIS Neuropathy Impairment Score, NYHA New York Heart Association, siRNA small interfering ribonucleic acid, TTR transthyretin, UPCR urine protein/creatinine ratio. aStage 1 (ambulatory without assistance) or stage 2 (ambulatory with assistance). bIn the event of UPCR ≥ 1000 mg/g, eligibility may be confirmed by a repeat random urine test with UPCR < 1000 mg/g or a quantitative total urine protein measurement of < 1000 mg/24 h. cChronic Kidney Disease Epidemiology Collaboration equation 1 formula