Abstract
Background
Anterior cruciate ligament (ACL) tear is the most frequent ligamentous injury of the knee joint. Autografts of hamstring (HS) or quadriceps tendons (QT) are used for primary ACL reconstruction. In this study, we planned to examine whether harvesting an HS graft is related to a deficit in dynamic knee stabilisation and strength revealed by dynamic valgus as compared with QT graft or the uninjured leg. Furthermore, if this deficit exists, is it compensated by higher neuromuscular activity of the quadriceps muscle?
Materials and methods
Adult patients who had undergone ACL reconstruction with QT or HS autografts were included in this two-armed cohort study. Clinical outcome was assessed by clinical data analysis, physical examination and the Lysholm Score and Knee Injury and Osteoarthritis Score (KOOS). In addition, gait analysis and non-invasive surface electromyography were performed.
Results
A complete data set of 25 patients (QT: N = 8, HS: N = 17) was analysed. There was no significant demographic difference between the groups. Time between surgery and follow-up was significantly longer for the QT group. Significant differences regarding clinical outcome were not found between the treated and untreated leg or between the two groups, with excellent scores at the time of follow-up. Gait analysis revealed no significant differences of varus–valgus angles. Significant differences in surface electromyography were only found in the QT group with increased vastus medialis obliquus activity of the treated legs (p < 0.01).
Conclusions
Our results suggest that harvesting of HS grafts for primary ACL reconstruction will not lead to a medial collapse and consequently impaired medial stabilisation of the knee when compared with QT grafts.
Level of evidence
IV.
Keywords: ACL reconstruction, Hamstring graft, Quadriceps tendon graft, Gait analysis, Surface electromyography
Introduction
Among those factors that impact clinical outcome after ACL reconstruction, graft selection might be the most critical yet controversial [1–3]. HS grafts are widely used and likely the current gold standard for primary ACL reconstruction [4–6]. The underlying rationale for this trend is a reliable and more anatomic fixation and a low rate of complications and resulting comorbidities [6, 7]. However, harvesting HS grafts eventually results in the impairment of flexor muscle strength and dynamic medial stabilisation [8–11]. This was demonstrated when HS grafts were compared with BPTB grafts [12] and QT grafts [13]. Since the ischiocrural muscles function as agonist of the ACL and as medial stabilisers, in theory, harvesting HS grafts could be a risk factor for recurrent injury. In contrast, patients who received HS grafts for primary ACL reconstruction had better extensor muscle strength compared with BPTB and QT collectives [13, 14]. Autologous HS and BPTB grafts have been thoroughly investigated, and both have been shown to provide comparable results regarding restoration of knee joint function and clinical outcome [15, 16]. Differences, however, seem to be slight, and both types of grafts are viable options for primary ACL reconstruction [15–20]. Another safe, versatile and suitable graft is the QT [21–23]. QT grafts in primary ACL reconstruction provide similar results to HS grafts without affecting comorbidities [24]. Lee et al. [10] showed that using either double-bundle HS or QT autografts resulted in similar functional outcome, yet better flexor muscle strength was observed in the QT group.
Although literature is comprehensive, the discussion about graft choice remains controversial [1, 2, 19, 21, 25]. Muscle strength might be a critical predictor of clinical outcome [26] and return to sports or preinjury activity levels. There is evidence that harvesting HS grafts weakens the agonists of the ACL and the MCL [9, 11], with the explanation that the hamstrings function as dynamic medial stabilisers [8]. Harvesting of HS grafts seems to result in strength deficits with knee flexion and inferior dynamic stability [12, 14, 27, 28]. In contrast, harvesting of QT or BPTB grafts weakens the quadriceps muscle.
Therefore, we planned to examine whether harvesting an HS graft is related to a deficit in dynamic knee stabilisation and strength revealed by dynamic valgus as compared with QT graft or the uninjured leg. Furthermore, the study was designed to reveal a potential strength deficit by compensatory neuromuscular activity of the quadriceps muscle.
Materials and methods
Inclusion criteria
Female and male patients, 18 years and older, who had received either a QT or HS autograph for ACL reconstruction in our department with a minimum follow-up period of 24 months were included in this cohort study. Additionally, to be included in this study, the patients were required to have the capability to walk on the treadmill for as long as 10 min. Poor compliance, previous surgeries on the knee, relevant comorbidities such as MCL injury or meniscal lesions at time of injury, and current injuries at time of follow-up were exclusion criteria. Concomitant injuries and pathologies representing exclusion criteria were monitored by physical examination and magnetic resonance imaging (MRI). To ensure consensus, participants had to complete a general anamnesis questionnaire.
Ethical approval
All procedures involving human participants were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. The study was approved by the institutional ethical committee (AZ 14-296). Informed consent was obtained from all individuals, and participation was voluntary.
Demographics
Overall, between 2008 and 2014, 202 patients were identified who had had ACL reconstruction in our department (QT: N = 51; HS: N = 152). In total, 103 patients met the inclusion criteria (QT: N = 23; HS: N = 80). Twenty-five patients participated in gait analysis (QT: N = 8; HS: N = 17), and were eligible for statistical analysis (QT: N = 8, female: N = 4, male: N = 4; HS: N = 17, female: N = 8, male N = 9). There were no significant differences between the QT and HS treatment groups regarding age (QT: 23 ± 12.96 SD years; HS 37 ± 11.66 SD years; p = 0.3565), height (QT: 175.6 ± 10.89 SD cm; HS: 177.3 ± 8.48 SD cm; p = 0.7093), weight (QT: 74.5 ± 16.97 SD kg; HS: 78.54 ± 15.05 SD kg; p = 0.5754) and body mass index (BMI; QT: 24.09 ± 4.17 SD kg/m2; HS: 24.97 ± 4.39 SD kg/m2; p = 0.6374).
Clinical outcome
Clinical outcome assessment included measurement of the range of motion (ROM) and circumference of both the treated and contralateral intact leg. Leg circumference measurements were done according to the measuring sheet of the Deutsche Gesetzliche Unfallversicherung (DGUV) for lower extremities 20 cm and 10 cm above the joint line, at the middle of the patella, and 15 cm below the joint line. The smallest circumference of the lower leg was measured also. Prior to this, the dominant leg was identified. The anterior drawer test and the Lachman test were performed. The visual analogue scale (VAS) for pain level and the Lysholm and KOOS scores were used as patient-reported quantitative outcome measures. Graft failures and treatment associated complications were documented.
Gait analysis
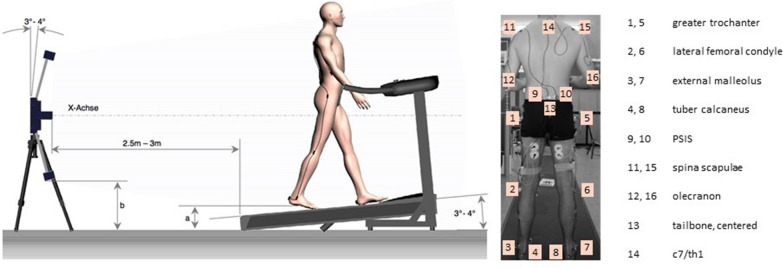
For the measurement of varus–valgus angles, the GaitLab system (Lutz Mechatronic Technology, Innsbruck, Austria) was used. The LUKOtronic Motion-Capture-Unit (MCU200) is equipped with infrared markers and sensors that allow for three-dimensional gait analysis. Infrared markers were fixed on the subjects as depicted in Fig. 1 (right). Subjects were guided through the system and were allowed to adapt to the Calles treadmill (Sprintex, Kleines Wiesental, Germany) for a couple of minutes (Fig. 1, left). Subsequently, measurements were started and conducted at both 4.5 and 6.0 km/h for as long as 5 min. Angles were measured between marker No. 1–3 (left leg) and No. 5–7 (right leg). The GaitLab software subdivides the individual gait cycle into 50 specific moments and enables calculation of varus (negative values) and valgus (positive values) angles. Initial contact (heel strike) was defined as the start of gait cycle, and terminal swing was defined as individual blank value. To enable inter-individual comparability, varus–valgus angles of the terminal swing phase were subtracted from the values of interest at loading response, terminal stance and mid-stance.
Fig. 1.
Schematic figure showing the layout of the gait analysis with the LUKOtronic Motion-Capture-Unit MCU200 (left); Picture with all the infrared marker positions shown from behind (right)
Surface electromyography
Non-invasive surface electromyography was used to measure neuromuscular activity of the semitendinosus muscle, the VMO and the VLO. Electrodes (Version 2.11, Shimmer, Dublin, IR) were placed according to the recommendations of the Surface ElectroMyoGrapy for the Non-Invasive Assessment of Muscles (SENIAM) projects. Sensors and wires were fixed thoroughly to prevent motion artefacts. Prior to measurements, normalisation of maximum voluntary contraction (MVC) was performed to enable inter-individual and quantitative comparability [29]. Neuromuscular activity (µV) of VMO and VLO was measured using triple maximum isometric extension of the leg for 10 s (defined resistance). The semitendinosus muscle was tested the same way, but by triple maximum isometric flexion of the leg. In real time, data were transferred to a laptop via Bluetooth, using the Multi Shimmer Sync software to display the raw data and the DIAdem software (National Instruments, Munich, GER) to analyse the data.
Statistical analysis
Statistical power analysis was based on a study by Tashman et al. [30]. The authors investigated n = 6 patients after ACL reconstruction using gait analysis for the measurement of valgus adduction [°] as major outcome measure. They found 2.8 ± 1.6° valgus adduction in ACL reconstructed limbs compared with healthy contralateral controls (0 ± 1.6°), resulting in an effect size of 1.75 (Cohens d). An a priori power analysis was conducted with G*Power (t-test: α = 0.05, power (1−β) = 0.85, effect size d = 1.75) [46]. According to our hypothesis and based on the referred study, we calculated a minimum of n = 7 per group. The data shown represent the means ± standard deviation (SD). The Mann–Whitney U test was run on clinical outcome data (Lysholm score and KOOS). Gait analysis and surface electromyography were analysed by the robust t-test of Yuen for differences and the two one-sided test (TOST) for equality using RStudio (Version 0.99.879; Boston, MA, USA). Gender differences were described by the χ2 test. Demographic data was compared by t-tests. The level of significance was set at p < 0.05.
Results
Clinical outcome
The mean follow-up of the QT group was 68.6 months, and of the HS group 31.1 months. At individual follow-ups, there were no significant differences in range-of-motion measurements between the treated and the contralateral intact legs and between the two treatment groups. The same was true for leg circumference measurements (p = 0.9673–0.1039). Pain based on VAS was 0.75 ± 1.39 SD in the QT group and 0.35 ± 0.86 SD in the HS group. The difference between the two groups was not statistically significant (p = 0.4746). Functional scores for both groups were excellent at time of follow-up (QT: Lysholm: 86.88 ± 10.09 SD, KOOS: 91.32 ± 8.44 SD; HS: Lysholm: 92.06 ± 9.32 SD, KOOS: 91.04 ± 8.22 SD). The overall difference between functional scores and differences between subcategories of select scores were not statistically significant (overall Lysholm score p = 0.18; overall KOOS score p = 0.821), except for a slight but significant advantage for the HS group for the Lysholm score item ‘climbing stairs’ (QT: 9.0 ± 1.85 SD; HS: 10 ± 0.0 SD; p = 0.04).
Gait analysis
Gait analysis revealed no significant differences of varus–valgus angles when comparing treated and contralateral intact legs. This was independent of the choice of transplant (QT or HS). These findings applied for loading response, mid-stance and terminal stance both at 4.5 km/h and 6.0 km/h. For detailed yield and p-values, see Table 1. When comparing the two different treatment groups (QT versus HS), there were no significant differences in varus–valgus angles measured at loading response, mid-stance and terminal stance both at 4.5 km/h and 6.0 km/h. For detailed yield and p-values, see Table 2. This was further evaluated, and a significant equality between the treated QT and HS group in varus–valgus angles measured at mid-stance and terminal stance both at 4.5 km/h (p = 0.0011; p = 0.0103) and 6.0 km/h (p = 0.0002; p = 0.0132) could be proven (Fig. 2).
Table 1.
Mean values and standard deviations of the varus–valgus angles for the treated and contralateral intact knees in the QT and HS group during the loading response, mid-stance and terminal stance at 4.5 km/h and 6.0 km/h
| Treated | Control | N | p | ||
|---|---|---|---|---|---|
| QT | |||||
| 4.5 km/h | Loading response | −1.48 ± 2.09 | 0.81 ± 0.99 | 8 | 0.65 |
| Mid-stance | −1.13 ± 1.56 | −0.84 ± 1.38 | 8 | 0.9 | |
| Terminal response | −1.02 ± 1.28 | −1.06 ± 1.66 | 8 | 0.93 | |
| 6.0 km/h | Loading response | −0.29 ± 0.77 | −0.49 ± 0.67 | 8 | 0.63 |
| Mid-stance | −0.03 ± 0.89 | −0.1 ± 0.18 | 8 | 0.99 | |
| Terminal response | −0.06 ± 1.87 | −0.18 ± 1.54 | 8 | 0.95 | |
| HS | |||||
| 4.5 km/h | Loading response | −0.16 ± 0.92 | −0.15 ± 0.94 | 17 | 0.2 |
| Mid-stance | −0.36 ± 0.93 | −0.1 ± 1.08 | 17 | 0.17 | |
| Terminal response | −0.83 ± 1.27 | −0.54 ± 1.11 | 17 | 0.5 | |
| 6.0 km/h | Loading response | −0.18 ± 0.92 | 0.14 ± 0.87 | 16 | 0.28 |
| Mid-stance | −0.03 ± 0.9 | 0.36 ± 0.95 | 16 | 0.09 | |
| Terminal response | −0.1 ± 1.54 | 0.55 ± 1.17 | 16 | 0.23 | |
Table 2.
Mean values and standard deviations of the varus–valgus angles for the treated knee in the QT and HS group during the loading response, mid-stance and terminal stance at 4.5 km/h and 6.0 km/h
| QT | HS | |||||
|---|---|---|---|---|---|---|
| Treated | N | Treated | N | p | ||
| 4.5 km/h | Loading response | −1.48 ± 2.09 | 8 | −0.16 ± 0.92 | 17 | 0.2388 |
| Mid-stance | −1.13 ± 1.56 | 8 | −0.36 ± 0.93 | 17 | 0.5058 | |
| Terminal response | −1.02 ± 1.28 | 8 | −0.83 ± 1.27 | 17 | 0.7319 | |
| 6.0 km/h | Loading response | −0.29 ± 0.77 | 8 | −0.18 ± 0.92 | 16 | 0.8109 |
| Mid-stance | −0.03 ± 0.89 | 8 | −0.03 ± 0.9 | 16 | 0.4902 | |
| Terminal response | −0.06 ± 1.87 | 8 | −0.1 ± 1.54 | 16 | 0.7572 | |
Fig. 2.
Boxplot charts comparing the varus–valgus angles between the QT (red) and HS (green) group during mid- and terminal stance at 4.5 km/h and 6.0 km/h. All four boxplot charts show significant equality between the treated knees of both groups
Surface electromyography
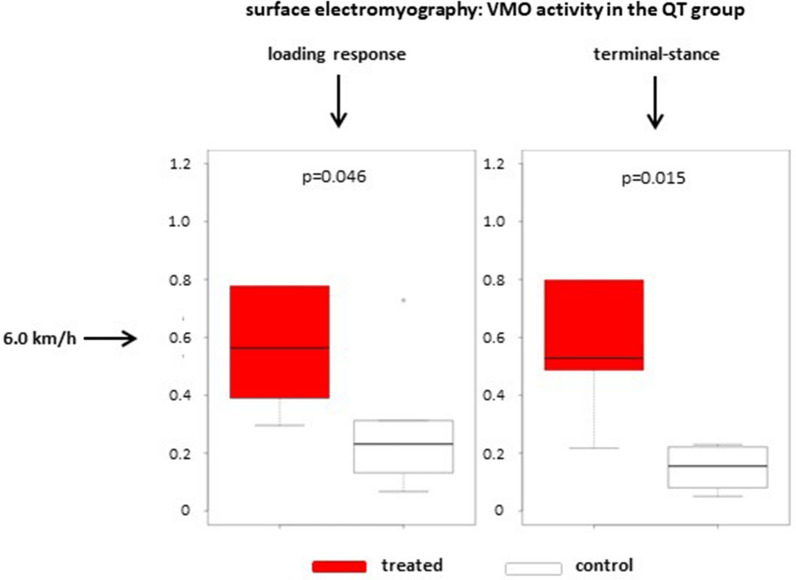
Surface electromyography was used to measure neuromuscular activity of the semitendinosus muscle, the VMO and the VLO. At 4.5 km/h, five QT cases and six HS cases were analysed, and six QT cases and seven HS cases were analysed at 6.0 km/h. In the QT group, there was one significant difference between the treated and contralateral intact legs with respect to VMO activity measured at loading response and terminal stance at 6.0 km/h (loading response: p = 0.046; terminal-stance: p = 0.015, Fig. 3). There were no other significant differences in the QT group. In the HS group, surface electromyography did not reveal any significant differences when comparing treated and contralateral intact legs, three different muscles and different stance phases. When comparing the treated legs of the QT and the HS groups, we found a significant difference (p = 0.0097) in VMO activity at terminal stance at 6.0 km/h (Fig. 4) with increased activity in the QT group and normal activity comparing in the HS group comparing the treated and contralateral intact legs. Other differences were not statistically significant.
Fig. 3.
Boxplot charts showing VMO activity (µV) for the treated and contralateral side in the QT group during loading response and terminal stance at 6.0 km/h. In both phases, there is a significant difference between the treated and contralateral intact side
Fig. 4.
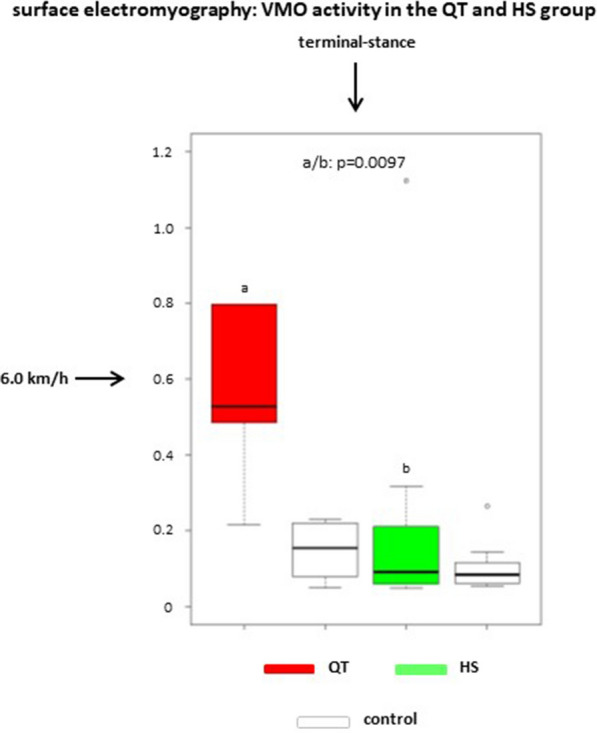
Boxplot chart showing VMO activity (µV) for the treated and contralateral side in the QT and HS groups during terminal stance at 6.0 km/h. There is a significant difference between the QT and HS groups regarding the treated legs
Discussion
Biomechanical studies implementing gait analysis have primarily focused on sagittal knee joint kinematics of ACL deficient and reconstructed knees [31, 32]. Data on dynamic changes in frontal knee joint angles comparing two different grafts have been analysed less often [31–33]. Reconsideration of dynamic stability after ACL reconstruction is not trivial because a dynamic valgus is a crucial predictor for clinical outcome and the return to sports or preinjury activity [26]. According to Abrams et al., harvesting HS grafts may result in strength deficits with knee flexion compared with BPTB-treated collectives [12]. Mohammadi et al. showed that HS grafts for ACL reconstruction performed better in terms of quadriceps strength in comparison with patients in the BPTB group [14]. Patients after ACL reconstruction had reduced quadriceps and hamstring strength and inferior dynamic stability, including hop performance and jump-landing strategy [14, 27, 28]. There is evidence that harvesting HS grafts weakens the agonists of the ACL and the main medial stabilizer MCL [9, 11], with the explanation that the hamstrings function as dynamic medial stabilisers [8]. Dynamic valgus indicates impaired medial stabilisation and consequently higher risk for re-injury. Comparing two different surgical techniques helps in deciding whether the current gold standard, HS graft [4], particularly in athletes [5], or other well-known grafts such as QT or BPTB grafts should be selected [2, 3].
In our study, both ACL reconstruction techniques (QT and HS) provided excellent clinical outcome, which is in line with other studies [34–37]. Gait analysis also proved both ACL reconstruction techniques to be successful. We could not find any significant difference between the QT and the HS groups. Moreover, we could not find any difference in frontal knee joint kinematics during walking compared with healthy contralateral controls. This is worth mentioning because prevailing dynamic valgus might have indicated muscular deficits due to the choice of implant. As for our hypothesis, this was true neither for the QT nor the HS group.
We could not confirm that the anticipated HS graft-related strength deficit would be compensated by higher neuromuscular activity of the quadriceps muscle. The opposite was true as treated legs in the QT group revealed a significantly higher VMO activity compared with healthy contralateral legs in the QT group and treated legs in the HS group. In contrast, surface electromyography in the HS group did not reveal any significant differences when comparing treated and contralateral intact legs. A higher neuromuscular activity of the VMO at terminal stance was found in the QT group, whereas Perry et al. described that there is usually no VMO activity at terminal stance in healthy collectives [38].
Xergia et al. summarized that isokinetic muscle strength deficits after ACL reconstruction are linked to the location of the donor site [39]. Ageberg et al. suggested that the strength deficit of the hamstrings and the lower hamstring-to-quadriceps ratio after HS harvesting will impair dynamic knee joint stabilisation [8]. Other studies showed that there is a strength deficit after harvesting BPTB or HS grafts even 5 years post ACL reconstruction [26, 40]. This evidence conflicts with our results, as enhanced neuromuscular activity of thigh muscles or dynamic valgus was not observed in the HS group, indicating either compensation of strength deficit or lack of medial stabilisation. A mild varus kinematic rather than dynamic valgus in both ACL reconstruction groups was found. Although mild varus kinematic cannot generally be equated to varus thrust, it is suspected to be a major reason for ACL reconstruction failure [41] and should be subject to further investigation, with respect to collective specific re-rupture rates.
We found increased VMO activity in the QT group, in line with a publication by Iriuchishima et al. [42], which showed quadriceps hypotrophy within 6 months after surgery, although hypotrophy had recovered after 12 months. This is remarkable because the mean follow-up of our QT group was 68.6 months compared with 31.1 months in the HS group. Thus, we could have expected a recovery especially of the QT group as described by Iriuchishima et al. [42]. Higher neuromuscular activity can also be interpreted as a compensatory mechanism for the strength deficit yet is not equal to muscle strength. In contrast, Bryant et al. concluded that subjects after ACL reconstruction demonstrated enhanced motor unit recruitment reflective of reduced quadriceps muscle fibre atrophy. This is coupled with increased quadriceps strength and musculotendinous stiffness of the lower limb musculature [43].
In conclusion, our results suggest that harvesting HS grafts for primary ACL reconstruction may not affect dynamic medial stabilisation. Our results are supportive of the use both of QS and of HS grafts for primary ACL reconstruction [36, 44]. However, graft fixation, gender, additional injuries and donor site morbidity have to be considered when deciding which graft to choose [20, 45]. Shortcomings of the present study are the small population, which is primarily due to the lifelike heterogeneity and concomitant injuries particularly of the MCL, which are prevalent in approximately one-third of all ACL cases. These cases had to be excluded. Moreover, dynamic valgus is multifactorial, and hip abductor, knee rotation and foot pronation issues should also be considered. Cadaveric cutting studies may have the potential to answer this eventually.
Acknowledgements
We are obliged to K. Waizner and M. Noodt for substantial technical and statistical support.
Abbreviations
- ACL
Anterior cruciate ligament
- QT
Quadriceps tendon
- HS
Hamstrings
- VMO
Vastus medialis obliquus
- BPTB
Bone patella tendon bone
- AZ
Aktenzeichen
- SD
Standard deviation
- BMI
Body mass index
- ROM
Range of motion
- KOOS
Knee injury and osteoarthritis outcome score
- VAS
Visual analogue scale
- VLO
Vastus lateralis obliquus
Authors’ contributions
All authors read and approved the manuscript. J.S.: study design, data analysis, statistics, graphs, manuscript writing; T.B.: study design, trials and examinations, data collection and assessment; R.W.: data analysis, statistics, gait analysis; A.P.S.: study design, data analysis, manuscript writing; J.G.: examinations, data collection and assessment; R.O.: study design, examinations, trials, data collection, manuscript writing. All authors read and approved the final manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL. The authors did not receive grants or outside funding in support of their research or preparation of this manuscript.
Availability of data and materials
The data sets used and/or analysed during the current study are available from the corresponding author on reasonable request. Besides, all data generated or analysed during this study are included in this published article.
Declarations
Ethics approval and consent to participate
The study was approved by the institutional ethical committee (AZ 14-296). Informed consent was obtained from all individuals, and participation was voluntary. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Consent for publication
Not applicable.
Competing interests
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
10/31/2021
Open access funding note missed in the original publication. Now, it has been added in the Funding section.
Contributor Information
J. Schagemann, Email: jan.schagemann@web.de
T. Koebrich, Email: koebrich.tobias@posteo.de
R. Wendlandt, Email: wendlandt@biomechatronics.de
A. P. Schulz, Email: schulz@biomechatronics.de
J. Gille, Email: justus_gille@usa.net
R. Oheim, Email: r.oheim@uke.de
References
- 1.Mohtadi NG, Chan DS, Dainty KN, et al. Patellar tendon versus hamstring tendon autograft for anterior cruciate ligament rupture in adults. Cochrane Library. 2011 doi: 10.1002/14651858.CD005960.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Petersen W, Benedetto K. Verschiedene Techniken zur Ersatzplastik des vorderen Kreuzbands. Arthroskopie. 2013;26:6–11. doi: 10.1007/s00142-012-0718-8. [DOI] [Google Scholar]
- 3.Shaerf DA, Pastides PS, Sarraf KM, et al. Anterior cruciate ligament reconstruction best practice: a review of graft choice. World J Orthop. 2014;5:23–29. doi: 10.5312/wjo.v5.i1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Domnick C, Garcia P, Raschke MJ, et al. Trends and incidences of ligament-surgeries and osteotomies of the knee: an analysis of German inpatient records 2005–2013. Arch Orthop Trauma Surg. 2017;137:989–995. doi: 10.1007/s00402-017-2704-0. [DOI] [PubMed] [Google Scholar]
- 5.Petersen W, Zantop T. Return to play following ACL reconstruction: survey among experienced arthroscopic surgeons (AGA instructors) Arch Orthop Trauma Surg. 2013;133:969–977. doi: 10.1007/s00402-013-1746-1. [DOI] [PubMed] [Google Scholar]
- 6.Pinczewski L, Roe J, Salmon L. Why autologous hamstring tendon reconstruction should now be considered the gold standard for anterior cruciate ligament reconstruction in athletes. Br J Sports Med. 2009;43:325–327. doi: 10.1136/bjsm.2009.058156. [DOI] [PubMed] [Google Scholar]
- 7.Yasuda K, Tsujino J, Ohkoshi Y, et al. Graft site morbidity with autogenous semitendinosus and gracilis tendons. Am J Sports Med. 1995;23:706–714. doi: 10.1177/036354659502300613. [DOI] [PubMed] [Google Scholar]
- 8.Ageberg E, Roos HP, Silbernagel KG, et al. Knee extension and flexion muscle power after anterior cruciate ligament reconstruction with patellar tendon graft or hamstring tendons graft: a cross-sectional comparison 3 years post surgery. Knee Surg Sports Traumatol Arthrosc. 2009;17:162–169. doi: 10.1007/s00167-008-0645-4. [DOI] [PubMed] [Google Scholar]
- 9.Herbort M, Michel P, Raschke MJ, et al. Should the ipsilateral hamstrings be used for anterior cruciate ligament reconstruction in the case of medial collateral ligament insufficiency? Biomechanical investigation regarding dynamic stabilization of the medial compartment by the hamstring muscles. Am J Sports Med. 2017;45:819–825. doi: 10.1177/0363546516677728. [DOI] [PubMed] [Google Scholar]
- 10.Lee JK, Lee S, Lee MC. Outcomes of anatomic anterior cruciate ligament reconstruction: bone–quadriceps tendon graft versus double-bundle hamstring tendon graft. Am J Sports Med. 2016;44:2323–2329. doi: 10.1177/0363546516650666. [DOI] [PubMed] [Google Scholar]
- 11.Maletis GB, Cameron SL, Tengan JJ, et al. A prospective randomized study of anterior cruciate ligament reconstruction: a comparison of patellar tendon and quadruple-strand semitendinosus/gracilis tendons fixed with bioabsorbable interference screws. Am J Sports Med. 2007;35:384–394. doi: 10.1177/0363546506294361. [DOI] [PubMed] [Google Scholar]
- 12.Abrams GD, Harris JD, Gupta AK, et al. Functional performance testing after anterior cruciate ligament reconstruction: a systematic review. Orthop J Sports Med. 2014;2:2325967113518305. doi: 10.1177/2325967113518305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Friedmann-Bette B, Profit F, Gwechenberger T, et al. Strength training effects on muscular regeneration after ACL reconstruction. Med Sci Sports Exerc. 2018;50:1152–1161. doi: 10.1249/MSS.0000000000001564. [DOI] [PubMed] [Google Scholar]
- 14.Mohammadi F, Salavati M, Akhbari B, et al. Comparison of functional outcome measures after ACL reconstruction in competitive soccer players: a randomized trial. JBJS. 2013;95:1271–1277. doi: 10.2106/JBJS.L.00724. [DOI] [PubMed] [Google Scholar]
- 15.Li S, Chen Y, Lin Z, et al. A systematic review of randomized controlled clinical trials comparing hamstring autografts versus bone-patellar tendon-bone autografts for the reconstruction of the anterior cruciate ligament. Arch Orthop Trauma Surg. 2012;132:1287–1297. doi: 10.1007/s00402-012-1532-5. [DOI] [PubMed] [Google Scholar]
- 16.Poehling-Monaghan KL, Salem H, Ross KE, et al. Long-term outcomes in anterior cruciate ligament reconstruction: a systematic review of patellar tendon versus hamstring autografts. Orthop J Sports Med. 2017;5:2325967117709735. doi: 10.1177/2325967117709735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sajovic M, Vengust V, Komadina R, et al. A prospective, randomized comparison of semitendinosus and gracilis tendon versus patellar tendon autografts for anterior cruciate ligament reconstruction: five-year follow-up. Am J Sports Med. 2006;34:1933–1940. doi: 10.1177/0363546506290726. [DOI] [PubMed] [Google Scholar]
- 18.Paulos LE, Wnorowski DC, Greenwald AE. Infrapatellar contracture syndrome: diagnosis, treatment, and long-term followup. Am J Sports Med. 1994;22:440–449. doi: 10.1177/036354659402200402. [DOI] [PubMed] [Google Scholar]
- 19.Xie X, Liu X, Chen Z, et al. A meta-analysis of bone–patellar tendon–bone autograft versus four-strand hamstring tendon autograft for anterior cruciate ligament reconstruction. Knee. 2015;22:100–110. doi: 10.1016/j.knee.2014.11.014. [DOI] [PubMed] [Google Scholar]
- 20.Samuelsen BT, Webster KE, Johnson NR, et al. Hamstring autograft versus patellar tendon autograft for ACL reconstruction: is there a difference in graft failure rate? A meta-analysis of 47,613 patients. Clin Orthop Relat Res. 2017;475:2459–2468. doi: 10.1007/s11999-017-5278-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Slone HS, Romine SE, Premkumar A, et al. Quadriceps tendon autograft for anterior cruciate ligament reconstruction: a comprehensive review of current literature and systematic review of clinical results. Arthroscopy. 2015;31:541–554. doi: 10.1016/j.arthro.2014.11.010. [DOI] [PubMed] [Google Scholar]
- 22.Mulford JS, Hutchinson SE, Hang JR. Outcomes for primary anterior cruciate reconstruction with the quadriceps autograft: a systematic review. Knee Surg Sports Traumatol Arthrosc. 2013;21:1882–1888. doi: 10.1007/s00167-012-2212-2. [DOI] [PubMed] [Google Scholar]
- 23.Lund B, Nielsen T, Faunø P, et al. Is quadriceps tendon a better graft choice than patellar tendon? A prospective randomized study. Arthroscopy. 2014;30:593–598. doi: 10.1016/j.arthro.2014.01.012. [DOI] [PubMed] [Google Scholar]
- 24.Cavaignac E, Coulin B, Tscholl P, et al. Is quadriceps tendon autograft a better choice than hamstring autograft for anterior cruciate ligament reconstruction? A comparative study with a mean follow-up of 3.6 years. Am J Sports Med. 2017;45:1326–1332. doi: 10.1177/0363546516688665. [DOI] [PubMed] [Google Scholar]
- 25.Spindler KP, Kuhn JE, Freedman KB, et al. Anterior cruciate ligament reconstruction autograft choice: bone-tendon-bone versus hamstring: does it really matter? A systematic review. Am J Sports Med. 2004;32:1986–1995. doi: 10.1177/0363546504271211. [DOI] [PubMed] [Google Scholar]
- 26.Moisala A-S, Järvelä T, Kannus P, et al. Muscle strength evaluations after ACL reconstruction. Int J Sports Med. 2007;28:868–872. doi: 10.1055/s-2007-964912. [DOI] [PubMed] [Google Scholar]
- 27.Petersen W, Taheri P, Forkel P, et al. Return to play following ACL reconstruction: a systematic review about strength deficits. Arch Orthop Trauma Surg. 2014;134:1417–1428. doi: 10.1007/s00402-014-1992-x. [DOI] [PubMed] [Google Scholar]
- 28.Kline PW, Morgan KD, Johnson DL, et al. Impaired quadriceps rate of torque development and knee mechanics after anterior cruciate ligament reconstruction with patellar tendon autograft. Am J Sports Med. 2015;43:2553–2558. doi: 10.1177/0363546515595834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hermens HJ, Freriks B, Disselhorst-Klug C, et al. Development of recommendations for SEMG sensors and sensor placement procedures. J Electromyogr Kinesiol. 2000;10:361–374. doi: 10.1016/S1050-6411(00)00027-4. [DOI] [PubMed] [Google Scholar]
- 30.Tashman S, Collon D, Anderson K, et al. Abnormal rotational knee motion during running after anterior cruciate ligament reconstruction. Am J Sports Med. 2004;32:975–983. doi: 10.1177/0363546503261709. [DOI] [PubMed] [Google Scholar]
- 31.Gao B, Zheng NN. Alterations in three-dimensional joint kinematics of anterior cruciate ligament-deficient and-reconstructed knees during walking. Clin Biomech. 2010;25:222–229. doi: 10.1016/j.clinbiomech.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 32.Gokeler A, Benjaminse A, van Eck CF, et al. Return of normal gait as an outcome measurement in ACL reconstructed patients. A systematic review. Int J Sports Phys Therapy. 2013;8:441–451. [PMC free article] [PubMed] [Google Scholar]
- 33.Butler RJ, Minick KI, Ferber R, et al. Gait mechanics after ACL reconstruction: implications for the early onset of knee osteoarthritis. Br J Sports Med. 2009;43:366–370. doi: 10.1136/bjsm.2008.052522. [DOI] [PubMed] [Google Scholar]
- 34.Barenius B, Nordlander M, Ponzer S, et al. Quality of life and clinical outcome after anterior cruciate ligament reconstruction using patellar tendon graft or quadrupled semitendinosus graft an 8-year follow-up of a randomized controlled trial. Am J Sports Med. 2010;38:1533–1541. doi: 10.1177/0363546510369549. [DOI] [PubMed] [Google Scholar]
- 35.Leys T, Salmon L, Waller A, et al. Clinical results and risk factors for reinjury 15 years after anterior cruciate ligament reconstruction a prospective study of hamstring and patellar tendon grafts. Am J Sports Med. 2012;40:595–605. doi: 10.1177/0363546511430375. [DOI] [PubMed] [Google Scholar]
- 36.Paradowski PT, Bergman S, Sundén-Lundius A, et al. Knee complaints vary with age and gender in the adult population. Population-based reference data for the Knee injury and Osteoarthritis Outcome Score (KOOS) BMC Musculoskeletal Disord. 2006;7:38. doi: 10.1186/1471-2474-7-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schulz AP, Lange V, Gille J, et al. Anterior cruciate ligament reconstruction using bone plug-free quadriceps tendon autograft: intermediate-term clinical outcome after 24–36 months. Open Access J Sports Med. 2013;4:243–249. doi: 10.2147/OAJSM.S49223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Perry J, Burnfield JM. Gait analysis: normal and pathological function. New Jersey: SLACK Incorporated; 2010. [Google Scholar]
- 39.Xergia SA, McClelland JA, Kvist J, et al. The influence of graft choice on isokinetic muscle strength 4–24 months after anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2011;19:768–780. doi: 10.1007/s00167-010-1357-0. [DOI] [PubMed] [Google Scholar]
- 40.Lautamies R, Harilainen A, Kettunen J, et al. Isokinetic quadriceps and hamstring muscle strength and knee function 5 years after anterior cruciate ligament reconstruction: comparison between bone-patellar tendon-bone and hamstring tendon autografts. Knee Surg Sports Traumatol Arthrosc. 2008;16:1009–1016. doi: 10.1007/s00167-008-0598-7. [DOI] [PubMed] [Google Scholar]
- 41.van de Pol JG, Arnold MP, Verdonschot N, et al. Varus alignment leads to increased forces in the anterior cruciate ligament. Am J Sports Med. 2009;37:481–487. doi: 10.1177/0363546508326715. [DOI] [PubMed] [Google Scholar]
- 42.Iriuchishima T, Ryu K, Okano T, et al. The evaluation of muscle recovery after anatomical single-bundle ACL reconstruction using a quadriceps autograft. Knee Surg Sports Traumatol Arthrosc. 2017;25:1449–1453. doi: 10.1007/s00167-016-4124-z. [DOI] [PubMed] [Google Scholar]
- 43.Bryant AL, Kelly J, Hohmann E. Neuromuscular adaptations and correlates of knee functionality following ACL reconstruction. J Orthop Res. 2008;26:126–135. doi: 10.1002/jor.20472. [DOI] [PubMed] [Google Scholar]
- 44.Sharma A, Flanigan DC, Randall K, et al. Does gracilis preservation matter in anterior cruciate ligament reconstruction? A systematic review. Arthroscopy. 2016;32:1165–1173. doi: 10.1016/j.arthro.2015.11.027. [DOI] [PubMed] [Google Scholar]
- 45.Barenius B, Forssblad M, Engström B, et al. Functional recovery after anterior cruciate ligament reconstruction, a study of health-related quality of life based on the Swedish National Knee Ligament Register. Knee Surg Sports Traumatol Arthrosc. 2013;21:914–927. doi: 10.1007/s00167-012-2162-8. [DOI] [PubMed] [Google Scholar]
- 46.Faul F, Erdfelder E, Lang AG, et al. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39:175–191. doi: 10.3758/BF03193146. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data sets used and/or analysed during the current study are available from the corresponding author on reasonable request. Besides, all data generated or analysed during this study are included in this published article.