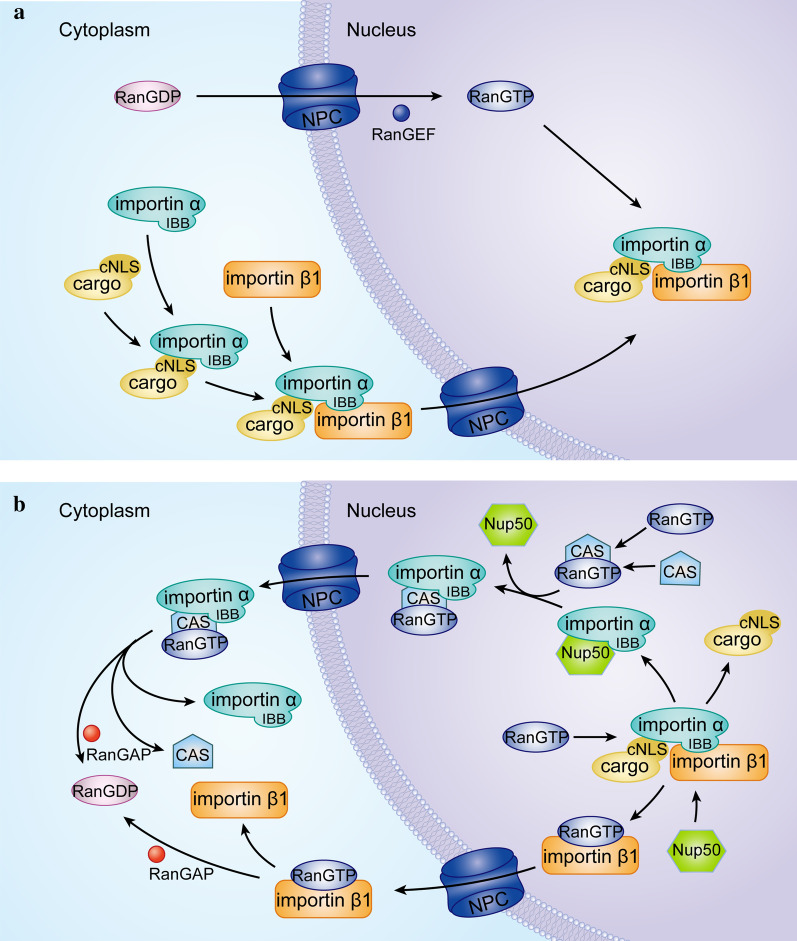
Fig. 1.
Schematic model of nucleoplasmic transport of cNLS-cargo protein. a Schematic model for cNLS-cargo protein import. The concentration of RanGDP protein in the cytoplasm is high, and cargo proteins with a cNLS are imported by the carrier importin β1, which binds them through the importin β1 binding (IBB) domain of the adaptor protein importin α to form the cNLS-cargo-importin α-importin β1 trimer under the action of various factors. Importin β1 directs importin α to the nuclear pore complex (NPC), and transfers the trimer complex to the nucleus by interacting with multiple phenylalanine-glycine (FG) repeats on the Nups [63]. The compartmentalization of RanGAP (GTPase activating protein) and RanEGF (guanine nucleotide exchange factor) is the basis of the proposed predominance of RanGDP in the cytoplasm and RanGTP in the nucleus [64]. b Schematic diagram of the cycle model of protein molecules related to nuclear transport. After passing through the NPC, the binding of RanGTP to importin β1 leads to the dissociation of importin β1 from the IBB domain of importin α. Nucleoporins such as Nup50 catalyze cargo dissociation and function as a molecular ratchet that prevents futile cycles. After dissociation, importin α is exported from the nucleus by CAS in conjunction with RanGTP. The importin β1-RanGTP complex is also returned to the cytoplasm, ready for reuse in the next round of transport