FIG 1.
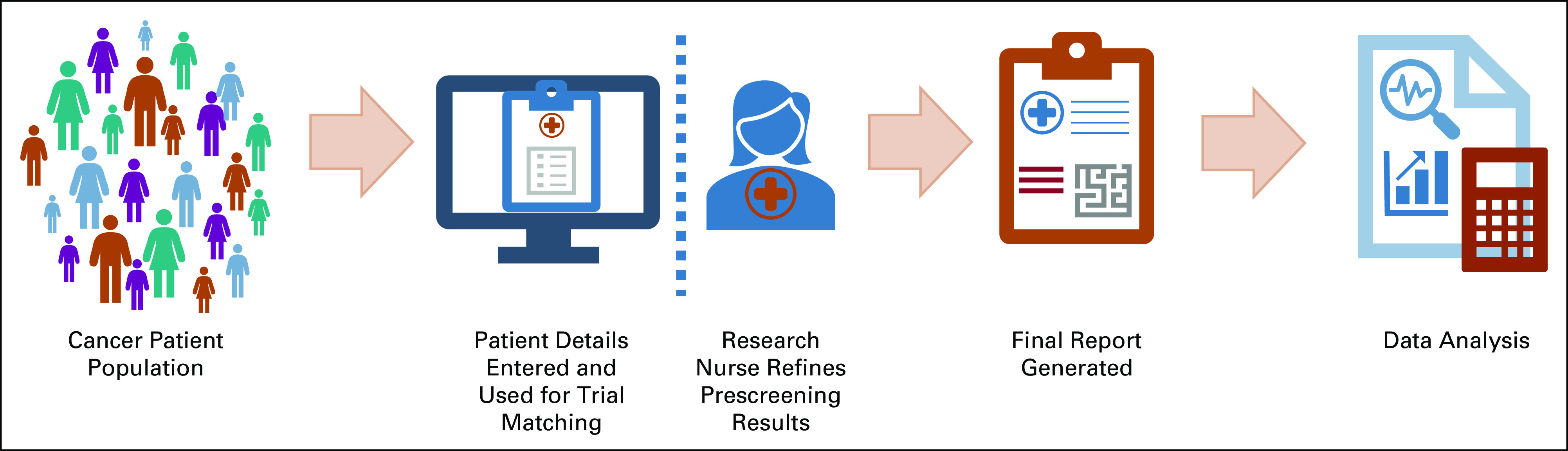
Schematic outlining the study workflow. Patients were identified based on the receipt of an NGS report, and their clinical reports were uploaded using a web-based user interface to PCTM. Relevant patient details such as vital status, diagnosis, date of diagnosis, and other biomarkers tested outside of the NGS test (eg, PD-L1 and IHC for ER or PR or HER2) were added manually. PCTM then generated a list of potential trials for each patient based on diagnosis and biomarker profile. Additional refinements to these trial results were applied using built-in multifaceted filtering for treatment setting and trial phase. Furthermore, a research nurse performed an initial manual prescreening to evaluate eligibility. The final list of eligible trials was compared against the initial recommendations by the PCTM software. ER, estrogen receptor; HER2, human epidermal growth factor receptor 2; IHC, immunohistochemistry; NGS, next-generation sequencing; PCTM, precision clinical trial matching; PD-L1, programmed death-ligand 1; PR, progesterone receptor.
