Figure 6. Putative TSE neoantigens and their correlation with immune response.
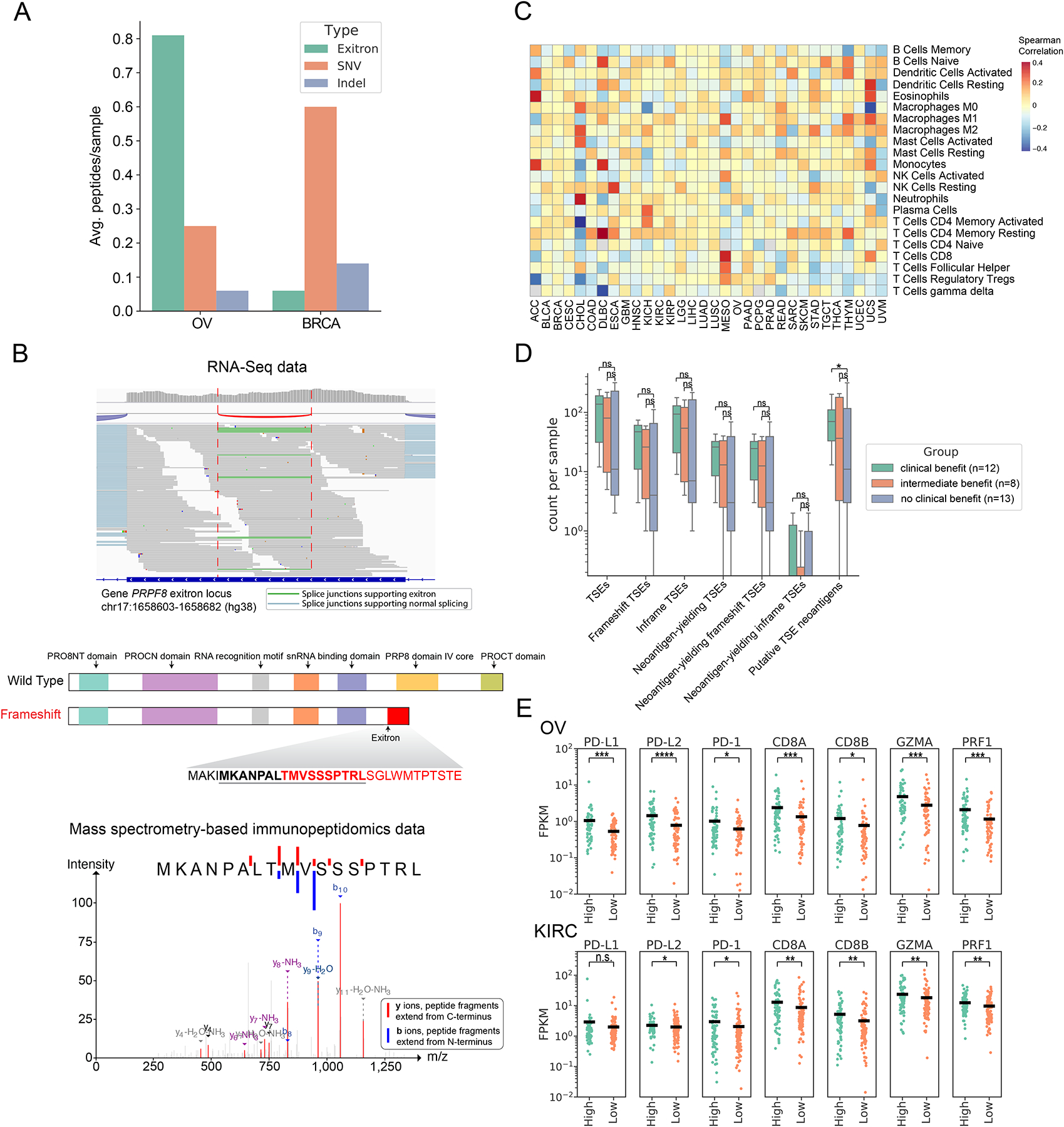
(A) Comparison of the contribution of TSE splicing, somatic SNVs and indels to CPTAC proteomic-confirmed putative neoantigens in BRCA and OV. (B) RNA-Seq data of ovarian cancer patient OvCa65 showed a 79bp exitron in PRPF8 exon 33 (top panel). Predicted functional domains disrupted by this frameshift exitron splicing event in PRPF8 (middle panel). A predicted neoantigen resulting from this frameshift exitron in PRPF8 was found by mass spectrometry to be presented in the corresponding immunopeptidome (bottom panel). (C) TSE neoantigen burden correlates with individual immune cell types in TCGA tumors. Values displayed are the Spearman correlation of immune cell fractions (rows) with neoantigen count within each tumor type (columns). Red indicates positive correlation (increasing proportion of indicated cell type with increasing neoantigen burden), and blue indicates negative correlation. (D) TSE neoantigen burden is associated with checkpoint inhibitor response in clear cell renal cell carcinoma (ccRCC). (E) Expression of T cell markers (PD-1, CD8A, CD8B), cytolytic activity markers (GZMA and PRF1) and immune-regulatory molecules (PD-L1 and PD-L2) in patients between top quartile TSE neoantigen load (named high group) and bottom quartile TSE neoantigen load (named low group) in OV and KIRC (n.s., not significant (p > 0.05), *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, Mann-Whitney rank test).
