Abstract
There are approximately 38 million people globally living with Human immunodeficiency virus 1 (HIV-1) and given the tremendous success of combination antiretroviral therapy (cART) this has dramatically reduced mortality and morbidity with prevention benefits. However, HIV-1 persists during cART within the human body and re-appears upon cART interruption. This HIV-1 reservoir remains a barrier to cure with cellular sites of viral persistence not fully understood. In this study we provide evidence corroborating a recently published article in STM demonstrating the role of platelets as a novel cellular disseminator of HIV-1 particles in the setting of viral suppression. Using classical transmission electron microscopy with and without immunogold labeling, we visualize HIV-1 in both platelets and monocytes in cART suppressed HIV donors. Our study suggests that due to the close proximity of platelets and monocytes an alternative life cycle of HIV-1 cycling within monocytes and platelets without the need of active replication under cART occurs. Our findings are supported by the lack of detectable HIV-1 particles in platelets derived from HIV uninfected donors or the ‘Berlin’ patient suggesting that platelets may serve as an underappreciated hidden bearer for HIV-1 and should be considered in HIV remission studies and trials.
To the editor
Combination antiretroviral therapy (cART) suppresses HIV-1 replication in people living with HIV (PLWH), which ultimately restricts HIV-1 transmission and the progression of AIDS-related disease. Virally suppressed PLWH, however, still exhibit resurgence of HIV when cART is interrupted [1]. The two ‘Boston patients’, after being diagnosed and treated for hematological tumors, underwent bone marrow transplantation with cells derived from HIV-uninfected donors [2]. Both individuals had a resurgence of blood viral loads within 12 or 32 weeks, indicating the existence of an active virus-producing reservoir separate from the bone marrow-derived cell lineages [2]. In view of the recently published article by Real et al. [3] demonstrating that human platelets can harbor replication-competent HIV-1 even in the context of cART, we wish to extend these findings here by illustrating the existence of monocyte-platelet-HIV particle interactions.
To identify HIV-1 particles we imaged immune cells, including monocytes and monocyte-derived macrophages, from PLWH on suppressive cART alongside healthy, HIV-uninfected individuals, and the ‘Berlin’ patient known to be the first patient cured from HIV-1 infection by both scanning and transmission electron microscopy (SEM and TEM; Donor characteristics in Supplementary Table 1). Our data revealed that platelets were present in every monocyte preparation, both in the proximity of and attached to monocytes (Figure 1A). Interestingly, adherent or ‘hitchhiking’ platelets were also present on monocyte-derived macrophages after being in culture for more than 7 days, a phenomenon we recently reported in a study independent from HIV-1 [4]. However, we were unable to detect major differences of platelet adherence to immune cells by visual examination between control and HIV-1 infected study participants, indicating that perhaps the limitations of SEM for these quantitative purposes, especially since monocyte-platelet aggregates are shown to be more frequent in PLWH compared to healthy controls [5]. When examining monocytes or macrophages by TEM we found a large number of platelets which were identified by their specific ultrastructural characteristics (glycogen pockets, the open canalicular system (OCS), and dense granules). We observed the appearance of virus-like particles present within the platelet population (Figure 1B), even though study participants were on cART. It is known that platelets possess the capability to capture and engulf HIV [6–8]. We also identified virus-like particles (VLP) within endosomal structures and in the OCS of platelets, as previously described [7]. Several observed VLPs displayed a dense core, which could indicate these particles are still undergoing maturation [9]. However, VLP ultrastructure imaged is in accordance with previous finding [3, 9–13]. Extension of pseudopods, a sign of platelet activation (Figure 1B, I arrowhead), and an OCS displaying dilation were also observed.
Figure 1: Hiding in plain sight – Platelets as carriers of HIV-1.
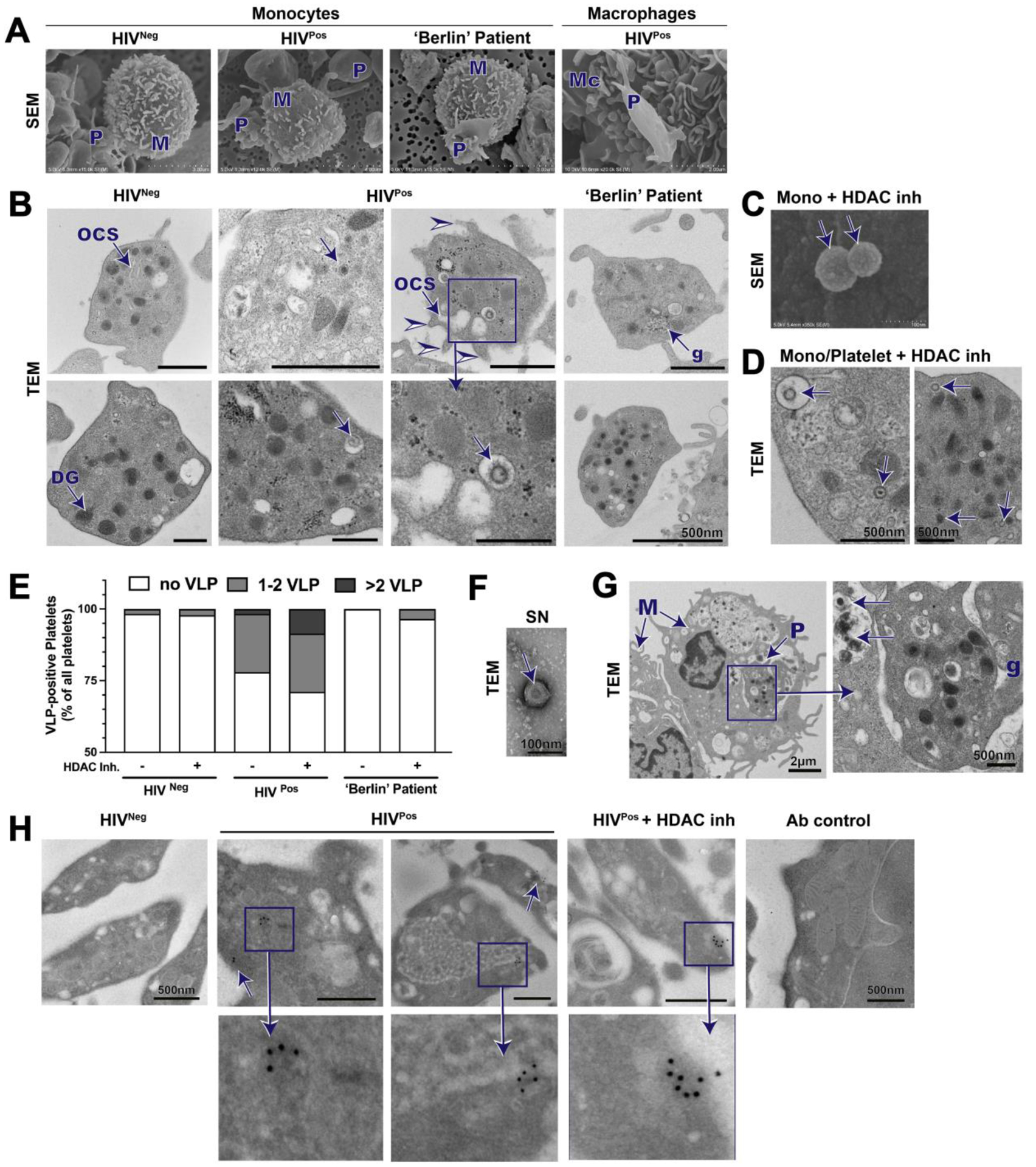
Platelets (P) adhering to monocytes (M) and macrophages (Mc) from healthy (HIVneg), suppressed HIV-1-infected individuals (HIVpos), and the ‘Berlin’ patient by SEM (A). TEM sections imaging monocyte preparations and platelets surrounding monocytes (B); platelet histological features: glycogen pockets (g), open canalicular system (OCS), and electron dense granules (DG). Viral particles (arrows) are detectable in platelets of HIVpos. Pseudopods on platelets (arrowheads) were apparent, indicative of an increased activated state. Virus-like particles detected on surface by SEM of HIVpos monocytes treated with HDAC inhibitors (inh) (C) and intracellularly as detected by TEM (D). (E) Quantification of platelets with visual VLPs from TEM images of monocyte/platelet preparations with control or HDAC inhibitor treatment. Platelets with VLP were counted and separated into two groups; platelets with 1–2 VLPs or with >2 VLPs. (F)Visualization of VLPs in supernatant (SN) of HDAC inh. treated monocyte/platelet preparations. (G) TEM imaging reveals signs of monocytes phagocytosing platelets. (H) p24 immunogold labeling of monocyte/platelet preparations. Positive p24 staining is indicated by arrows.
We next examined monocytes and macrophages from the ‘Berlin patient’, the first person to be cured of HIV-1 infection. The ‘Berlin’ patient received a bone marrow transplant for treatment of leukemia from a donor with a CCR5Δ32 mutation and has since remained free of active viral infection in spite of not being on cART [14]. Monocyte-platelet aggregates in the ‘Berlin’ patient sample preparations, detected by SEM, were similar to aggregates observed in HIV-infected and control samples (Figure 1A), but more importantly, viral particles were not detected within platelets or monocytes/macrophages, further substantiating our observation that particles identified by TEM are HIV (Figure 1B).
Additionally, we incubated monocytes from PLWH on suppressive cART with agents that reverse HIV latency, histone deacetylase (HDAC) inhibitors, and noted the appearance of virus-like particles on the surface of monocytes (Figure 1C), although this needs to be confirmed. Additionally, the frequency of virus-like particles within platelets increased significantly after HDAC inhibitor treatment (Figure 1D, E). Several virus-like particles were found within a single platelet, whereas under control conditions only a few platelets each contained a single virus-like particle (Figure 1E). Additionally, we were able to detect VLP in the supernatant of PLWH on cART by negative staining and TEM imaging (Figure 1F). Our data suggest that HDAC inhibition increases virus-like particle release within both monocytes and their ‘hitchhiking’ platelets, potentially indicating that monocytes may harbor HIV-1 and particles released by monocytes can be rapidly engulfed by platelets, presumably due to their close symbiotic contact. We also showed in our TEM preparations evidence of monocytes phagocytosing platelets (Figure 1G), a phenomenon previously described [15].
To further substantiate whether the observed particles were indeed HIV-1, we employed immunogold labeling with several antibodies from the NIH AIDS Reagents Program that target HIV-1-specific proteins. Platelets of PLWH on cART showed positive staining for p24 (size of 70–100 nm; Figure 1H) within monocyte/platelet cultures, as indicated by at least two gold particles being present and potentially forming a semi-circle. In contrast, secondary antibody control staining and p24 staining of HIV negative cells did not reveal p24 staining associated with VLPs.
Based on the findings of Real et al. publication and our group, we propose the following mechanisms regarding platelet-monocyte complexes in viral persistence (Figure 2): In untreated HIV-1, HIV-1 particles are taken up by various cell types like monocytes or megakaryocytes where HIV-1 will integrate into the cellular DNA and persist after cART [16, 17]. Additionally, HIV-1 is engulfed by platelets where it is not able to integrate into cellular DNA and remains sequestered therefore persisting in circulation evading immune surveillance. Due to the close contact of platelets with other cell types, including monocytes HIV-1 virus particle transfer between platelets and other cell types is possible. Once cART is initiated, active proliferation of HIV-1 is inhibited; however, the close proximity of HIV-1-containing platelets with monocytes may provide a suitable environment for phagocytosis of the platelets or through bridging conduits [18, 19] or nanotubes allowing for viral particle exchange potentially reseeding the virus in monocytes and monocyte-derived tissue macrophages, which allows for persistence of HIV-1 within circulating cells and tissues. Additionally, we cannot exclude the possibility that HIV-1 found in platelets might be derived from megakaryocytes, a second potential way of transmission as megakaryocytes have recently been found to be carrying replication competent HIV-1 even under cART [3]. We therefore highlight the importance of further research investigating HIV-1 presence in platelets and circulating immune cells in PLWH on cART. We would also like to advocate using multiple techniques, especially 3D electron microscopy techniques (e.g., FIB-SEM) with correlative immunofluorescence in timeline experiments to determine whether phagocytosis of HIV-1 into platelets or from platelets into monocytes actually occurs. Presented here are images using classical TEM, which does not allow for verification of whether the location of the particles/platelets is due to phagocytosis or merely being surrounded by the monocyte/platelet. We further highlight the importance of displaying results from presumably negative internal assay controls for immunogold labeling to clearly verify and validate the presence of HIV-1 particles.
Figure 2: Hiding in plain sight – Platelets as carriers of HIV-1.
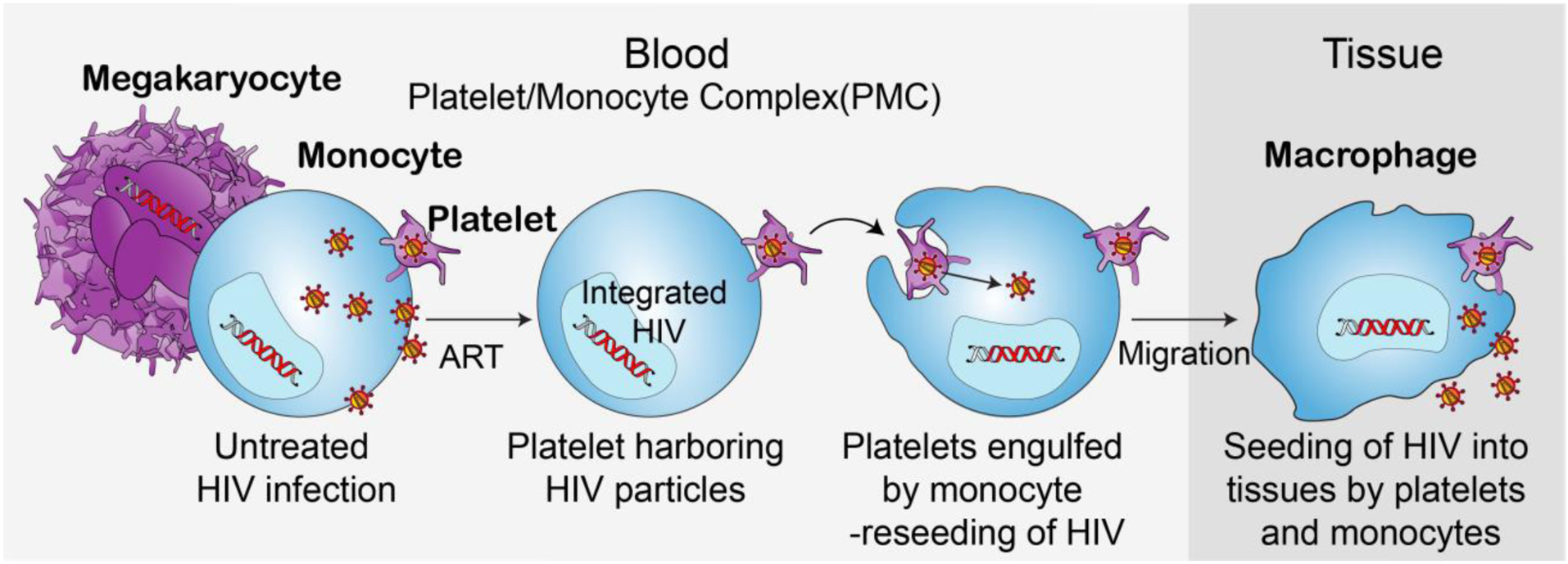
Proposed ‘life cycle’ of HIV-1 utilizing platelets as a carrier: Upon HIV-1 infection of a human body several cell types will be infected with HIV-1. HIV-1 integrates rather rapidly in the DNA of various cell types including megakaryocytes and monocytes. Additionally, in the scenario of untreated HIV-1 infection platelets can engulf HIV-1 particles. Due to the close contact of platelets and monocytes HIV-1 transfer between these two cell types, especially upon phagocytosis of dying platelets will occur. Upon start of cART treatment no active HIV-1 replication can occur, however, few platelets will still harbor HVI-1 particles and allow for an alternative ‘life’ cycle of HIV-1 under cART therapy allowing for circulation of active HIV-1 particles within monocytes, which subsequently can enter tissues, differentiate into macrophages carrying HIV-1 particles. It is likely that upon cell death within the tissue HIV-1 can either be distributed within the tissue or phagocytosed by other cell types entering another life cycle without the need of active replication, allowing HIV-1 lingering within cells and tissues able to infect various cell types.
The HIV-1 cell associated reservoir persists as resurgence has been observed in single-copy experiments even after 3–5 years of continuous cART [20]; therefore, further investigation to determine whether the HIV-1 particles we observed are replication competent and contribute to viral recrudescence. Recent literature have demonstrated that monocytes can serve as a HIV-1 reservoir capable of active viral replication [21–23] and these observations were more prominent in a subset of HIV infected donors [17]. Further research is therefore warranted to gain further insight into the ‘lifecycle’ of HIV latency and reservoir formation in monocytes, but also the role of platelets in the pathogenesis of comorbidities in PLWH on cART [24]. Platelets have been shown to drive inflammation and to potentially accelerate progression of various diseases [25, 26] as well as of HIV-1 [27]. Platelets engulfed by monocytes/macrophages have been reported to alter the phenotype of macrophage differentiation which can have multiple effects on many diseases [15]. Although our data are by no means complete and need to be confirmed using more powerful and specific imaging techniques, our findings demonstrate platelets could be an underappreciated bearer and conveyer of HIV-1 to myeloid cells which certainly needs to be considered and investigated in the global fight against HIV-1 infection.
Supplementary Material
Acknowledgments
The authors would like to acknowledge our study participants, including the Berlin Patient, all who are committed to advancing HIV cure research and the excellent scientific and clinical teams and research nurses of the Hawaii Center for AIDS. We thank the support from Mary Margaret Byron for assistance with specimen processing and scientific insights from Drs. J.A. Ananworanich (USMHRP) and V. Valcour (UCSF). This work was partially presented at the Keystone Symposia Mechanisms of HIV Persistence: Implications for a Cure in Boston, MA. We acknowledge the generosity of the National Institute of Health (NIH) AIDS Reagent Program for providing anti-P24 monoclonal antibodies. Finally, we would like to express our gratitude and sincere appreciation of Timothy Ray Brown, the Berlin patient, who recently passed away. He and the team of nurses and doctors around him dedicated their life to the further understanding of HIV/AIDS, the finding of a cure, and most importantly awareness for a disease which is still affecting millions of life worldwide. Our sincere condolences go to Timothy Ray Brown’s partner, family and friends.
Footnotes
Disclosure of interest
All authors read and approved the submission of the manuscript. LCN has received honorarium for participating in advisory meetings for Abbvie. All other authors report no potential conflicts of interest. The Social Determinants of Obesity and Cardiovascular Risk Laboratory is funded by Division of Intramural Research of the National Heart, Lung, and Blood Institute and Intramural Research Program of the National Institute on Minority Health and Health Disparities. Core facilities were supported by NIH grants P20GM103516, P20RR016453, G12RR003061, and G12MD007601. The Hitachi HT7700 TEM was acquired with NSF grant DBI-1040548. This project was also funded in part by the National Institute of Mental Health (award R01MH104141) to LCN.
Data Availability Statement
Data available upon reasonable request directed towards the corresponding author.
References
- [1].Deng K, Siliciano RF. HIV: Early treatment may not be early enough. Nature 2014;512:35–36. [DOI] [PubMed] [Google Scholar]
- [2].Henrich TJ, Hanhauser E, Marty FM, Sirignano MN, Keating S, Lee TH, Robles YP, Davis BT, Li JZ, Heisey A, et al. Antiretroviral-free HIV-1 remission and viral rebound after allogeneic stem cell transplantation: report of 2 cases. Annals of internal medicine 2014;161:319–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Real F, Capron C, Sennepin A, Arrigucci R, Zhu A, Sannier G, Zheng J, Xu L, Masse JM, Greffe S, et al. Platelets from HIV-infected individuals on antiretroviral drug therapy with poor CD4(+) T cell recovery can harbor replication-competent HIV despite viral suppression. Sci Transl Med 2020;12. Epub 2020/03/20. [DOI] [PubMed] [Google Scholar]
- [4].Baumer Y, Gutierrez-Huerta CA, Saxena A, Dagur PK, Langerman SD, Tamura K, Ceasar JN, Andrews MR, Mitchell V, Collins BS, et al. Immune cell phenotyping in low blood volumes for assessment of cardiovascular disease risk, development, and progression: a pilot study. J Transl Med 2020;18:29. Epub 2020/01/19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Liang H, Duan Z, Li D, Li D, Wang Z, Ren L, Shen T, Shao Y. Higher levels of circulating monocyte-platelet aggregates are correlated with viremia and increased sCD163 levels in HIV-1 infection. Cell Mol Immunol 2015;12:435–443. Epub 2014/08/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Chaipan C, Soilleux EJ, Simpson P, Hofmann H, Gramberg T, Marzi A, Geier M, Stewart EA, Eisemann J, Steinkasserer A, et al. DC-SIGN and CLEC-2 mediate human immunodeficiency virus type 1 capture by platelets. J Virol 2006;80:8951–8960. Epub 2006/08/31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Youssefian T, Drouin A, Masse JM, Guichard J, Cramer EM. Host defense role of platelets: engulfment of HIV and Staphylococcus aureus occurs in a specific subcellular compartment and is enhanced by platelet activation. Blood 2002;99:4021–4029. Epub 2002/05/16. [DOI] [PubMed] [Google Scholar]
- [8].Banerjee M, Huang Y, Joshi S, Popa GJ, Mendenhall MD, Wang QJ, Garvy BA, Myint T, Whiteheart SW. Platelets Endocytose Viral Particles and Are Activated via TLR (Toll-Like Receptor) Signaling. Arterioscler Thromb Vasc Biol 2020;40:1635–1650. Epub 2020/05/22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Roingeard P Viral detection by electron microscopy: past, present and future. Biol Cell 2008;100:491–501. Epub 2008/07/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Grigorov B, Muriaux D, Argirova R, Darlix J-L. New Insights into Human Immunodeficiency Virus—Type 1 Replication. Biotechnology & Biotechnological Equipment 2014;19:3–15. [Google Scholar]
- [11].Fontana J, Jurado KA, Cheng N, Ly NL, Fuchs JR, Gorelick RJ, Engelman AN, Steven AC. Distribution and Redistribution of HIV-1 Nucleocapsid Protein in Immature, Mature, and Integrase-Inhibited Virions: a Role for Integrase in Maturation. J Virol 2015;89:9765–9780. Epub 2015/07/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Yahi N, Sabatier JM, Baghdiguian S, Gonzalez-Scarano F, Fantini J. Synthetic multimeric peptides derived from the principal neutralization domain (V3 loop) of human immunodeficiency virus type 1 (HIV-1) gp120 bind to galactosylceramide and block HIV-1 infection in a human CD4-negative mucosal epithelial cell line. J Virol 1995;69:320–325. Epub 1995/01/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Ruffin N, Gea-Mallorqui E, Brouiller F, Jouve M, Silvin A, See P, Dutertre CA, Ginhoux F, Benaroch P. Constitutive Siglec-1 expression confers susceptibility to HIV-1 infection of human dendritic cell precursors. Proc Natl Acad Sci U S A 2019;116:21685–21693. Epub 2019/10/09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Hutter G, Nowak D, Mossner M, Ganepola S, Mussig A, Allers K, Schneider T, Hofmann J, Kucherer C, Blau O, et al. Long-term control of HIV by CCR5 Delta32/Delta32 stem-cell transplantation. N Engl J Med 2009;360:692–698. Epub 2009/02/14. [DOI] [PubMed] [Google Scholar]
- [15].Singhal R, Chawla S, Rathore DK, Bhasym A, Annarapu GK, Sharma V, Seth T, Guchhait P. Development of pro-inflammatory phenotype in monocytes after engulfing Hb-activated platelets in hemolytic disorders. Clin Immunol 2017;175:133–142. Epub 2017/01/01. [DOI] [PubMed] [Google Scholar]
- [16].Le Douce V, Herbein G, Rohr O, Schwartz C. Molecular mechanisms of HIV-1 persistence in the monocyte-macrophage lineage. Retrovirology 2010;7:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Massanella M, Bakeman W, Sithinamsuwan P, Fletcher JLK, Chomchey N, Tipsuk S, Chalermchai T, Routy JP, Ananworanich J, Valcour VG, et al. Infrequent HIV Infection of Circulating Monocytes during Antiretroviral Therapy. J Virol 2019;94. Epub 2019/10/11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Kadiu I, Gendelman HE. Macrophage bridging conduit trafficking of HIV-1 through the endoplasmic reticulum and Golgi network. J Proteome Res 2011;10:3225–3238. Epub 2011/05/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Hashimoto M, Bhuyan F, Hiyoshi M, Noyori O, Nasser H, Miyazaki M, Saito T, Kondoh Y, Osada H, Kimura S, et al. Potential Role of the Formation of Tunneling Nanotubes in HIV-1 Spread in Macrophages. The Journal of Immunology 2016:1500845. [DOI] [PubMed] [Google Scholar]
- [20].Palmer S, Maldarelli F, Wiegand A, Bernstein B, Hanna GJ, Brun SC, Kempf DJ, Mellors JW, Coffin JM, King MS. Low-level viremia persists for at least 7 years in patients on suppressive antiretroviral therapy. Proceedings of the National Academy of Sciences of the United States of America 2008;105:3879–3884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Avalos CR, Price SL, Forsyth ER, Pin JN, Shirk EN, Bullock BT, Queen SE, Li M, Gellerup D, O’Connor SL, et al. Quantitation of Productively Infected Monocytes and Macrophages of Simian Immunodeficiency Virus-Infected Macaques. J Virol 2016;90:5643–5656. Epub 2016/04/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Mitchell BI, Laws EI, Ndhlovu LC. Impact of Myeloid Reservoirs in HIV Cure Trials. Curr HIV/AIDS Rep 2019;16:129–140. Epub 2019/03/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Harrold SM, Wang G, McMahon DK, Riddler SA, Mellors JW, Becker JT, Caldararo R, Reinhart TA, Achim CL, Wiley CA. Recovery of replication-competent HIV type 1-infected circulating monocytes from individuals receiving antiretroviral therapy. AIDS Res Hum Retroviruses 2002;18:427–434. Epub 2002/04/18. [DOI] [PubMed] [Google Scholar]
- [24].Ho YC, Shan L, Hosmane NN, Wang J, Laskey SB, Rosenbloom DI, Lai J, Blankson JN, Siliciano JD, Siliciano RF. Replication-competent noninduced proviruses in the latent reservoir increase barrier to HIV-1 cure. Cell 2013;155:540–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Morrell CN, Aggrey AA, Chapman LM, Modjeski KL. Emerging roles for platelets as immune and inflammatory cells. Blood 2014;123:2759–2767. Epub 2014/03/04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Scheuermann L, Pei G, Domaszewska T, Zyla J, Oberbeck-Muller D, Bandermann S, Feng Y, Ruiz Moreno JS, Opitz B, Mollenkopf HJ, et al. Platelets Restrict the Oxidative Burst in Phagocytes and Facilitate Primary Progressive Tuberculosis. Am J Respir Crit Care Med 2020;202:730–744. Epub 2020/05/19. [DOI] [PubMed] [Google Scholar]
- [27].Rieg G, Yeaman M, Lail AE, Donfield SM, Gomperts ED, Daar ES, Hemophilia G, Development S. Platelet count is associated with plasma HIV type 1 RNA and disease progression. AIDS Res Hum Retroviruses 2007;23:1257–1261. Epub 2007/10/27. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


