Figure 2.
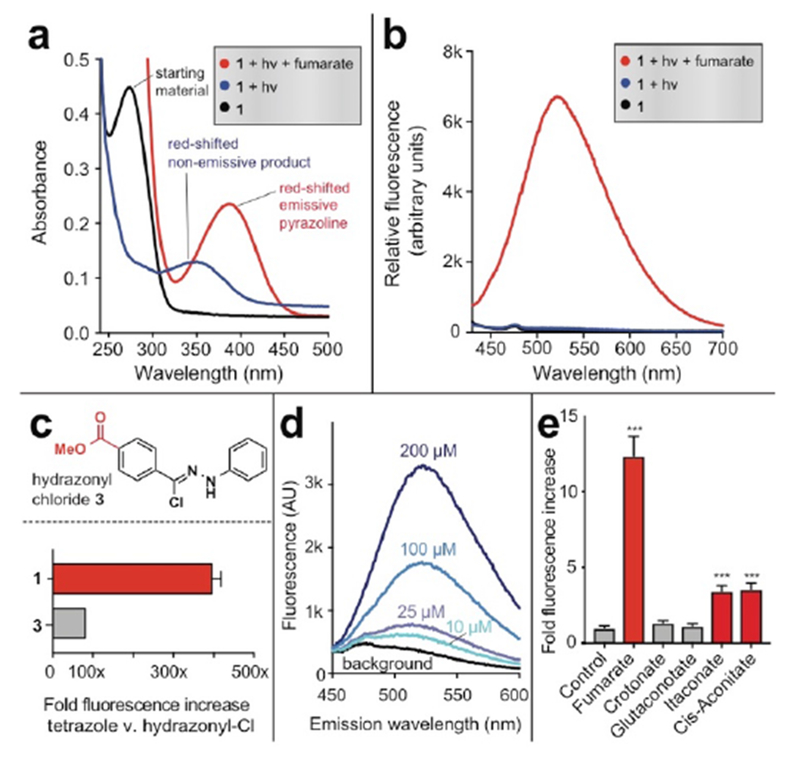
Fluorogenic detection of fumarate by tetrazole 1. (a) Absorbance spectra of tetrazole 1 following photolysis in the presence or absence of 10 mM fumarate. (b) Fluorescence spectra (λex = 410 nm) of tetrazole 1 following photolysis in the presence or absence of 10 mM fumarate. Reaction conditions: 100 μM tetrazole 1, 10 mM fumarate, 5% DMSO in PBS (pH 7.2), hν= 302 nm, 2 min. (c) Comparison of fluorescent detection of fumarate (10 mM) by tetrazole 1 (100 μM, hν=302 nm, 2 min) and previously described hydrazonyl chloride 3 (1 (100 μM, 1 h). (d) Limit of detection of fumarate by tetrazole 1. Data is representative of 3 replicates. (e) Fluorescent detection of other metabolite dipolarophiles by tetrazole 1 (λex = 410 nm, λem = 540 nm) Reaction conditions: 100 μM tetrazole 1, 200 μM metabolite, sodium phosphate buffer pH 7.0 (1:1), hν= 302 nm, 2 min. Statistical significance was determined by unpaired t test (n = 3, ***P < 0.001).
