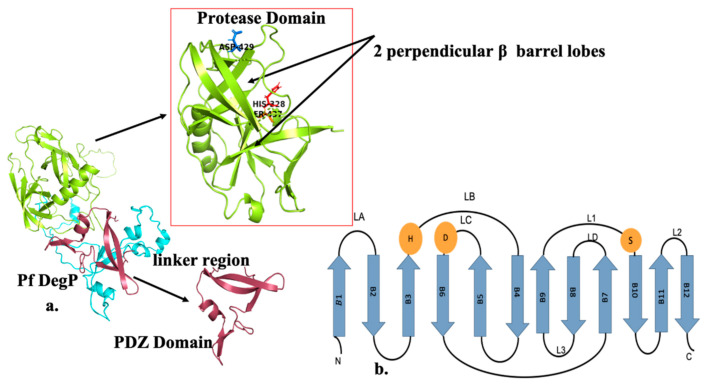
Figure 1.
(a) Domain organisation and representation of PfDegP protein: Domain architecture of DegP consists of three distinct domains which include an N-terminal region known to have regulatory functions, a conserved trypsin-like protease domain (PD) shown in lemon having two perpendicular β barrel lobes and along with the catalytic triad displayed as sticks: Histidine (His 328) shown in red color, Aspartate (Asp 429) shown in marine, Serine (Ser 437) depicted in orange, Linker Region shown in cyan and one PDZ domain in brick red (b) Secondary structure elements of the DegP protease domain. The protease domain depicts a chymotrypsin fold and the B strands (shown as arrows) forming N and C terminal B barrels. For the sake of simplicity, the alpha helices are not depicted here. Catalytic triad residues: H, D and S are shown as orange spheres. Based on the chymotrypsin nomenclature, the loops of the protease domain are named, loops LD, L1, L2 and L3 shown in the figure are known to be regulatory.