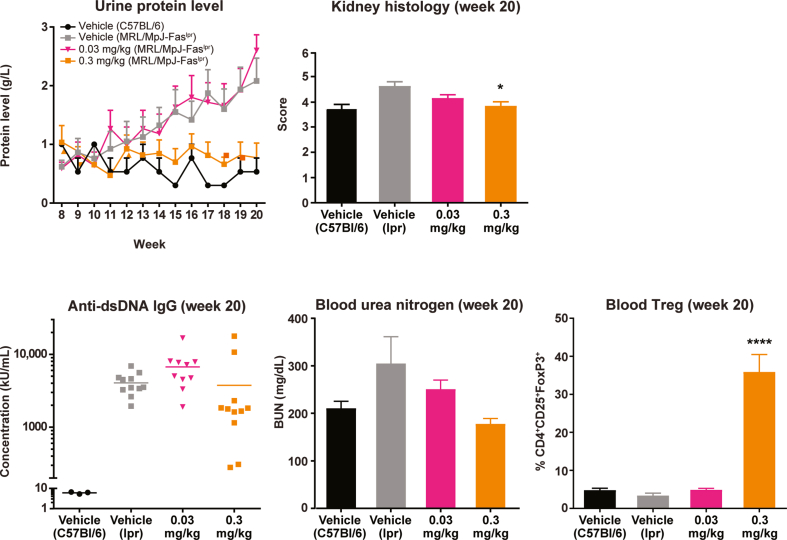
Fig. 7.
NKTR-358 is efficacious in a mouse model of SLE. Three groups of 15 MRL/MpJ-Faslpr mice were treated with vehicle or NKTR-358 at 0.03 or 0.3 mg/kg twice weekly for 12 weeks after an 8-week baseline period. A control group of three C57BL/6 mice received vehicle alone on the same dosing schedule. Urine was collected at baseline and weekly for protein analysis. Blood samples collected at baseline and at week 20 were analyzed for anti-dsDNA antibodies, blood urea nitrogen, and Treg populations. Kidney histology were evaluated at week 20. *p < 0.05; ****p < 0.0001. BUN, blood urea nitrogen; dsDNA, double-stranded DNA; IgG, immunoglobulin G; SLE, systemic lupus erythematosus; Treg, regulatory T cell.