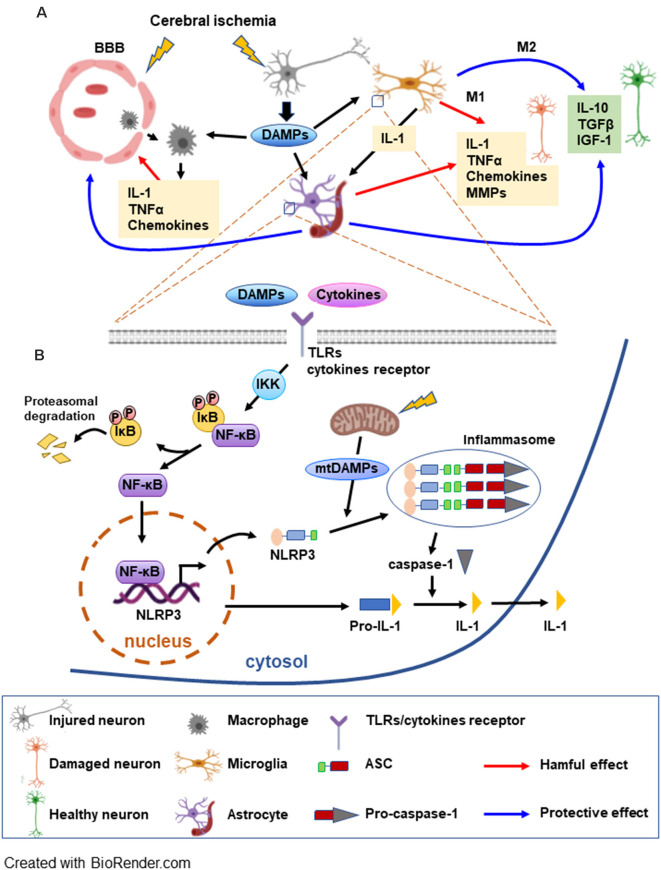
Figure 1.
Schematic overview of the postischemic inflammatory response and NF-κB signaling pathways mediating NLRP3 inflammasome activation. (A) Cerebral ischemia triggers the disruption of the blood brain barrier (BBB) allowing for the macrophages to migrate to the ischemic area. Injured neurons release DAMPs soon after the onset of ischemia, which can be detected by microglia, astrocytes, and macrophages initiating an inflammatory response. The inflammatory molecules released by the glia have deleterious as well beneficial effects on neurons. M1 microglia generate interleukin (IL)-1β and tumor necrosis factor (TNF) which further induce cytokine and chemokine release from astrocytes. M1 microglia also release MMPs, enhancing BBB disruption and worsening brain damage. A beneficial role of glia consists of producing anti-inflammatory cytokines and trophic molecules. Additionally, astrocytes can be involved in the repair of the BBB. (B) DAMPs and cytokines trigger TLR/cytokine receptor signaling, leading to NF-κB activation. NF-κB, a major factor in the inflammasome priming phase, is activated upon phosphorylation of the inhibitor IκB by IKK. NF-κB can then translocate to the nucleus to induce transcription of the inflammasome complex elements. DAMPs released by mitochondria (mtDAMPs) constitute the signal that triggers the assembly and activation of the inflammasome. Caspase-1 is activated by the inflammasome and can transform the inactive pro-IL-1 into active IL-1 which can be released (see text for abbreviations).