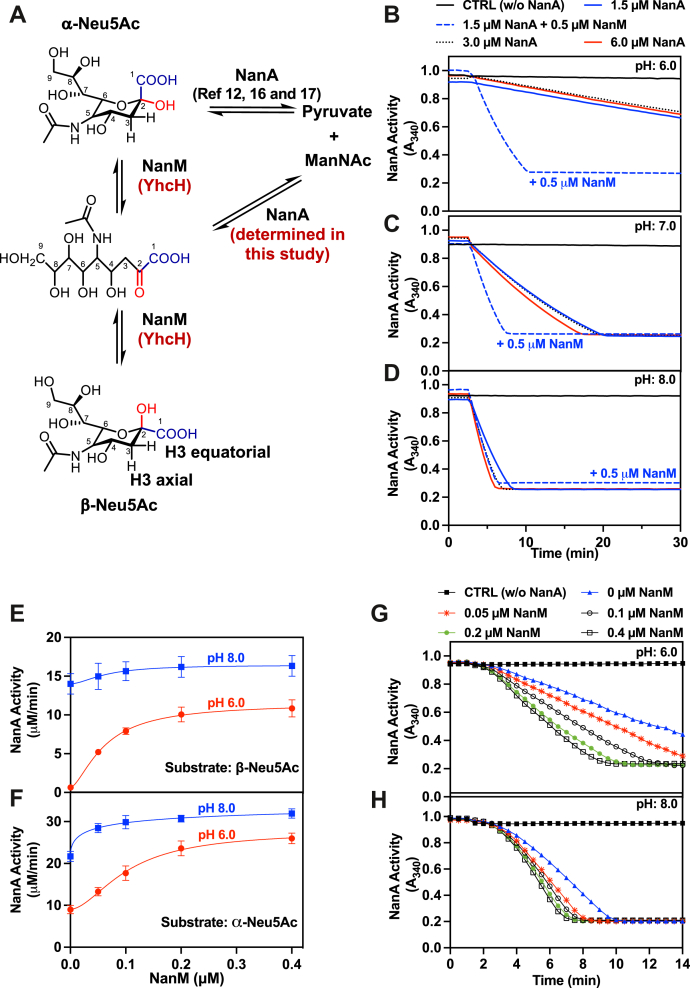
Figure 1.
Evidence that the Neu5Ac pyruvate aldolase NanA utilizes the open form of Neu5Ac.A, schematic representation of the various forms of Neu5Ac and the reactions catalyzed by the anomerase NanM and YhcH. The Neu5Ac aldolase NanA, which was previously thought to use the alpha-anomer, is now shown to use the open form of Neu5Ac. B–D, spectrophotometer tracings of Neu5Ac aldolase activity illustrating the effect of different concentrations of the aldolase NanA (1.5, 3.0, and 6.0 μM) and of the anomerase NanM at pH 6.0 (B), 7.0 (C), and 8.0 (D). NanM was added at 0.5 μM in some assays containing 1.5 μM NanA. Neu5Ac was present at 200 μM and NADH at 150 μM. The assays were conducted at 20 °C. E and F, effect of NanM to stimulate the utilization of α-Neu5Ac by NanA. Spectrophotometer tracings with 2 μM NanA and different concentrations of NanM at pH 6.0 (E) and 8.0 (F). At pH 6.0, α-Neu5Ac was generated from 3’ sialyllactose in the presence of 0.6 U/ml sialidase A. At pH 8.0, α-Neu5Ac was generated from 2,7-AN in the presence of 3 μM YjhC and 50 μM NAD+. The assays were conducted at 20 °C. G and H, effect of the concentration of NanM on the activity of NanA acting on either β-Neu5Ac (G) or α-Neu5Ac (H). The assays were conducted at 20 °C, at pH 6.0 or pH 8.0, with the indicated concentrations of NanM. NanA was added at 2 μM. Substrate concentration was 120 μM for Neu5Ac and 1 mM for 2,7-AN and 3’-sialyllactose. Results shown are means ± SD (n = 3).