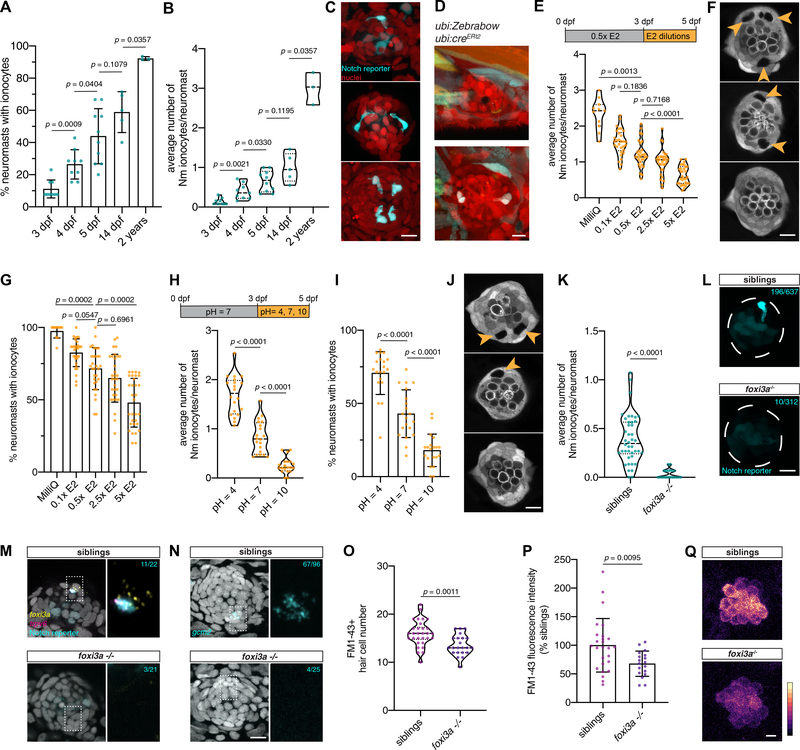
Figure 5: Nm ionocyte frequency is modulated by environmental changes.
(A) Percentage of neuromasts with Notch+ Nm ionocytes at 3 dpf (n = 9 fish, 117 neuromasts), 4 dpf (n = 9 fish, 125 neuromasts), 5 dpf (n = 10 fish, 162 neuromasts), 14 dpf (n = 5 fish, 49 neuromasts) and in 2-year old adult fish (n = 3 fish, 78 neuromasts, Mann-Whitney test). (B) Average number of Notch+ Nm ionocytes per neuromast at stages quantified in (A), (same n-numbers, Mann-Whitney test). (C) Representative maximum intensity projections of Nm ionocytes (arrowheads) at stages quantified in (A)-(B). (D) Maximum intensity projections of the same ubi:Zebrabow;ubi:creERt2 neuromast at 21 dpf (upper panel) and 28 dpf (lower panel) with newly appeared Nm ionocytes (arrowheads). (E) Nm ionocyte number in larvae incubated in control media (0.5x E2) or different concentrations (MilliQ, n = 11 fish, 154 neuromasts; 0.1x E2, n = 30 fish, 433 neuromasts; 0.5x E2, n = 31 fish, 436 neuromasts; 2.5x E2, n = 31 fish, 449 neuromasts; 5x E2, n = 31 fish, 454 neuromasts; Kruskal-Wallis ANOVA and Dunn’s post hoc test). (F) Representative images of neuromasts and Nm ionocytes (arrowheads) following incubation in E2 media dilutions. (G) Percentage of neuromasts with Nm ionocytes in larvae incubated in different E2 media dilutions (n-numbers as in (E), Kruskal-Wallis ANOVA and Dunn’s post hoc test). (H) Nm ionocyte frequency in acidic (pH = 4; n = 20 fish, 286 neuromasts), alkaline (pH = 10; n = 20 fish, 291 neuromasts) and neutral E2 media (pH = 7, n = 20 fish, 296 neuromasts; unpaired t-test). (I) Percentage of neuromasts with Nm ionocytes following incubation in E2 media of different pH (n-numbers as in (H), Mann-Whitney test). (J) Representative images of Nm ionocytes (arrowheads) as quantified in (I). (K) Average Nm ionocyte frequency in foxi3a−/− fish (n = 20 fish, 312 neuromasts) and their siblings (n = 42 fish, 637 neuromasts; Mann-Whitney test). (L) Representative maximum projections of Nm ionocytes (arrowheads) in foxi3a−/− fish and their siblings. (M) foxi3a and trpv6 HCR in foxi3a−/− fish (lower panel, n = 21 neuromasts, 4 fish) and their siblings (upper panel, n = 22 neuromasts, 7 fish). Cyan n-numbers indicate the number of neuromasts with trpv6+ Nm ionocytes. Magnification of boxed area is shown on the right. (N) gcm2 HCR in foxi3a−/− fish in the Notch reporter background (lower panel, n = 25 neuromasts, 3 fish) and their siblings (upper panel, n = 96 neuromasts, 12 fish). Cyan n-numbers indicate the number of neuromasts with gcm2+ Nm ionocytes. Magnification of boxed area is shown on the right. (O) FM1–43+ hair cell numbers in foxi3a−/− fish (n = 15 fish, 30 neuromasts) and their siblings (n = 11 fish, 22 neuromasts). (P) FM1–43 intensity of hair cells in foxi3a−/− fish (n = 9 fish, 18 neuromasts) compared to their siblings (n = 13 fish, 26 neuromasts; unpaired t-test). Data is shown as fluorescence intensity/background, normalized to the average of the siblings. (Q) FM1–43 dye uptake by hair cells in foxi3a−/− fish and their siblings, as quantified in (P). Scale bars = 5 μm.
All scale bars = 10 μm, unless otherwise noted. Error bars indicate standard deviation. Dashed lines in violin plots indicate the median, dotted lines indicate quartiles. Individual data points in A, B, E, G, H, I and K represent an average of all neuromasts quantified per fish. See also Figure Supplementary 4.