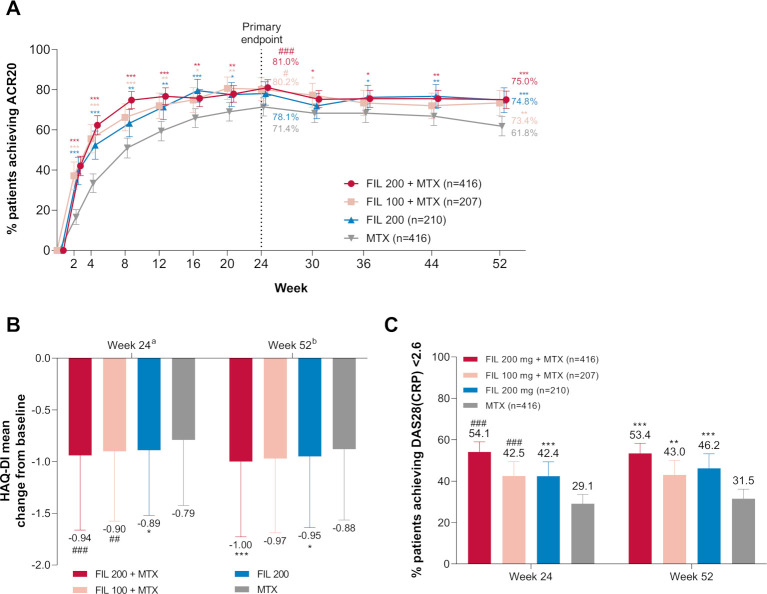
Figure 2.
Primary, key secondary, and other secondary efficacy outcomes: (A) proportion of patients who achieved ACR20 over time; (B) change from baseline in HAQ-DI at week 24 and week 52; (C) proportion of patients achieving DAS28(CRP) <2.6 at week 24 and week 52. ###p<0.001; ##p<0.01; #p<0.05. The difference between filgotinib 200 mg and MTX for ACR20 at week 24 was not significant (p=0.058). ***Exploratory p<0.001; **exploratory p<0.01; *exploratory p<0.05, for supportive analysis without adjustment for multiplicity. an=372, 190, 185, and 370 for FIL200+MTX, FIL100+MTX, FIL200, and MTX, respectively. bn=332, 169, 171, and 307 for FIL200+MTX, FIL100+MTX, FIL200, and MTX, respectively. Error bars represent 95% CI for proportions of patients and SD for mean. For HAQ-DI, p values are based on least-squares mean difference versus MTX. Supporting values for (A) are shown in online supplemental table S3. ACR20, 20% improvement in American College of Rheumatology criteria; DAS28(CRP), 28-joint Disease Activity Score with C-reactive protein; FIL, filgotinib; HAQ-DI, Health Assessment Questionnaire-Disability Index; MTX, methotrexate.