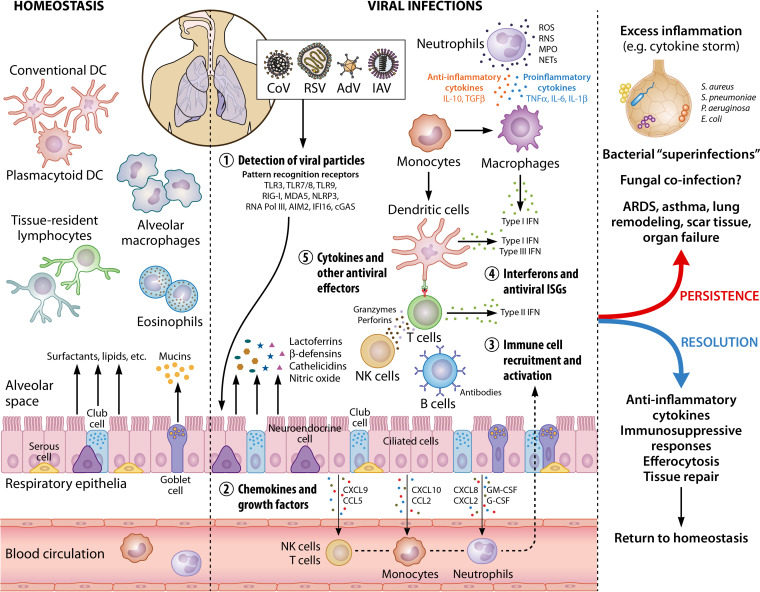
FIG 2.
Immune cell activation in the lungs during viral infections. In healthy lungs, the resident immune cells include primarily alveolar macrophages, conventional and plasmacytoid dendritic cells, and tissue-resident lymphocytes and eosinophils, which patrol the tissue for foreign threats. In addition, respiratory epithelial cells (e.g., club cells and goblet cells) secrete mucins, surfactants, and other molecules that preserve homeostasis, as well as maintain immune cells in their quiescent states. During viral infections, viruses are sensed by innate immune receptors (or pattern recognition receptors) which activate immune responses. Secretion of chemokines and growth factors by respiratory epithelium and resident immune cells leads to the phased recruitment and activation of neutrophils, monocytes, NK cells, and T cells. Type I (IFN-α/β) and type III (IFN-λ) IFNs are the primary cytokines produced upon viral detection, followed by other inflammatory cytokines, as well as ISGs. Other antiviral effectors produced by epithelial, endothelial, and immune cells include lactoferrins, β-defensins, ROS, and RNS. Innate immune cells (neutrophils, macrophages, and DCs) mediate the activation of the adaptive responses (NK cells, B cells, and T cells) that involve IFN-γ secretion, antibody production, and cytotoxic killing of infected cells. Persistence of a viral infection, as well as the accompanying antiviral immune responses, often leads to widespread lung damage and secondary complications such as systemic inflammation (due to dysregulated immune responses) and bacterial coinfections. A prolonged infection can result in oxygen deprivation, ARDS, asthma, remodeled lung structure (e.g., excess collagen deposition, thickening of basal membrane, and scar tissue formation), organ failure, and even death in extreme cases. Resolution of a respiratory viral infection by a kinetically controlled, successful immune activation and a reversal to homeostasis is facilitated by anti-inflammatory cytokines, immunosuppressive molecules, the removal of active, cytotoxic immune cells by efferocytosis, and extensive tissue repair. This image was created using BioRender (BioRender.com). DC, dendritic cells; CoV, coronavirus; RSV, respiratory syncytial virus; AdV, adenovirus; IAV, influenza virus; TLR, Toll-like receptor; RIG-I, retinoic-acid inducible gene I; MDA5, melanoma differentiation-associated protein 5; NLRP3, NOD-like receptor protein 3; RNA Pol III, RNA polymerase III; AIM2, absent in melanoma 2; IFI16, IFN-inducible protein 16; cGAS, cyclic GMP-AMP synthase; CXCL, C-X-C motif chemokine ligand; CCL, C-C motif chemokine ligand; GM-CSF, granulocyte-macrophage colony-stimulating factor; G-CSF, granulocyte colony-stimulating factor; NK cells, natural killer cells; IFN, interferon; ISG, interferon-stimulated genes; IL, interleukin; TNF, tumor necrosis factor; TGFβ, transforming growth factor β; ROS, reactive oxygen species; RNS, reactive nitrogen species; MPO, myeloperoxidase; NET, neutrophil extracellular traps.