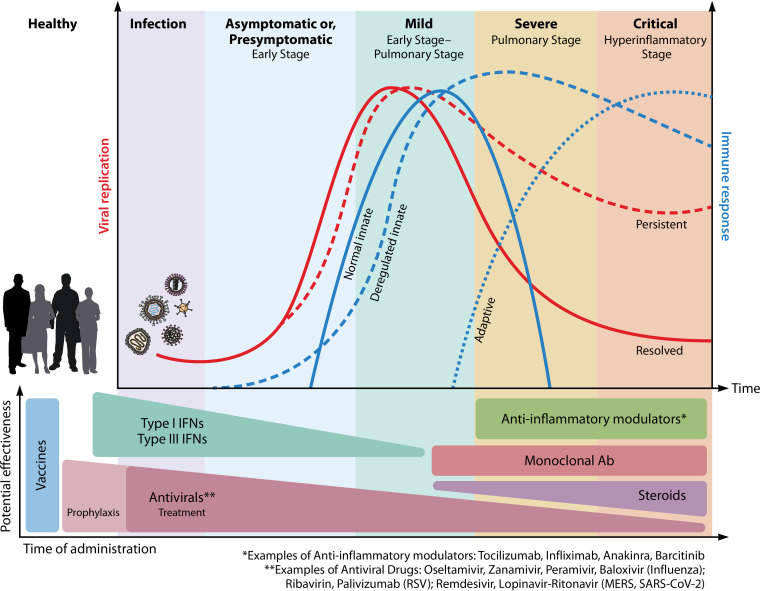
FIG 3.
Clinical interventions impacting immune responses and viral replication during respiratory viral infections. Several preventive and therapeutic interventions targeting immune responses are approved or currently being tested to combat virus-dependent immunopathology. After initial exposure to a respiratory virus, symptoms begin manifesting within a few days, depending on the virus replication kinetics. The clinical severity of disease can range from mild to moderate, severe, or critical (that define the early, pulmonary, or hyperinflammatory stages) and is often dictated by a persisting infection and/or an aberrant, uncontrolled antiviral immune response. Severe cases of viral infections frequently require hospitalization that can lead to intensive care and ventilation in patients with deteriorating lung functions or other associated systemic complications. In a healthy, uninfected individual, the administration of vaccines is the most effective route to prevent respiratory viral illnesses. Examples include annual vaccinations against the predicted evolving strains of influenza. Currently, several vaccines against COVID-19 have received emergency authorization around the world. Recent authorized SARS-CoV-2 vaccines (with published data from phase 3 clinical trials) include mRNA vaccines from Pfizer-BioNTech and Moderna, as well as an adenovirus-based vaccine from Oxford-AstraZeneca. Prophylaxis with antiviral drugs is another preventive measure for unimmunized children or immunocompromised individuals, or against viruses for which there are no vaccines (e.g., RSV). Prophylactics include oral oseltamivir phosphate for influenza and palivizumab for RSV, and there are considerations for developing nasally administered IFNs for prophylaxis against SARS-CoV-2. In asymptomatic patients or presymptomatic patients with early detection or with mild disease, early complementary type I or type III IFN administration can be beneficial. In severe cases of lung infection, multiple anti-inflammatory immunomodulators such as neutralizing antibodies or inhibitors are used to supplement the antiviral therapies to prevent and mitigate cytokine storm. While mild respiratory illnesses can be relieved with over-the-counter fever-reducing drugs, pathogen-specific antiviral drugs are used in cases of moderate to critical disease, either singularly or in combination with other therapeutic interventions. In COVID-19, patients have also benefited from treatment with antiviral monoclonal antibody cocktails (e.g., bamlanivimab by Eli Lilly or casirivimab and imdevimab by Regeneron) and with convalescent plasma from recovered individuals with high antibody titers. Early administration of the antivirals displays better efficiency in viral clearance. In critical patients with hyperimmune responses and ARDS, treatment with corticosteroids (e.g., dexamethasone) relieves respiratory distress. The effectiveness of steroids in the first phases of a viral infection is controversial, since they may hamper the establishment of a properly effective antiviral response. The image was created using BioRender.