Figure 3.
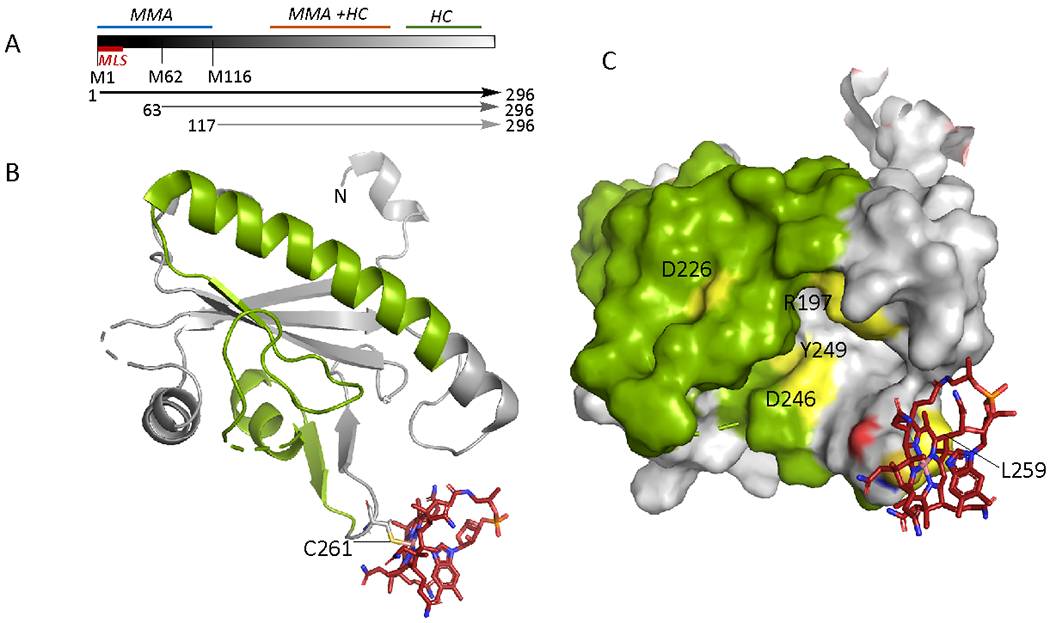
Organization and structure of human CblD. A. Schematic representation showing locations of the mitochondrial leader sequence (MLS) and the alternate initiation sites at Met-62 and Met-116, leading to proteins of varied lengths (arrows). Mutations in the N-terminal region (1-115) result in isolated methylmalonic aciduria (MMA), mutations near the C-terminus (197-259) lead to isolated homocystinuria (HC), while mutations in the central region lead to both. B Structure of CblD (PDB: 6X8Z) starting at residue 133 (denoted as N) with thiolato-cob(III)alamin bound at Cys-261. The secondary structure elements in which mutations lead to isolated homocystinuria are shown in green C. Surface representation of CblD showing the location of individual residues (yellow) in the 197-259 region (green) that lead to isolated homocystinuria when mutated.
