Abstract
Efficacy, safety and tolerability of lacosamide in the treatment of status epilepticus are well described. However, other evidence of its pharmacologic profile in elderly patients with other comorbidities seems warranted. We describe the case of an 80 year-old woman with an history of arterial hypertension, ischemic cardiomyopathy, COPD, CKD, previous laryngeal cancer, a stoma positioning for diverticular disease and previous surgery for a left frontal meningioma. Since then, the patient developed focal epilepsy and she was on levetiracetam and valproic acid therapy. The patient was admitted to our department for a focal status epilepticus characterized by non-fluent aphasia and right facio-brachial clonic movements. She also presented with aspiration pneumonia and started intravenous antibiotic treatment. After failure of a first-line antiepileptic drug, lacosamide intravenous treatment was started, with complete reversal of the clinical picture. EEG then showed focal slow waves mixed to interictal epileptiform discharges over the left fronto-temporal regions. The patient was then discharged home with an oral lacosamide treatment and at 3 months she was seizure-free. Our case report confirms the efficacy of lacosamide in status epilepticus, highlighting its safety and tolerability in an elderly and fragile patient with multiple comorbidities and drug therapy.
Keywords: Status epilepticus, lacosamide, comorbidities, AEDs
Introduction
Status epilepticus (SE) is a serious and life threatening neurological emergency defined as a condition of abnormally prolonged seizures that can result in long-term consequences, secondary to the failure of the mechanisms responsible for seizure termination or from the initiation of such mechanisms.
It can be classified using semiology, etiology, electroencephalographic correlates and age as diagnostic axes. From a clinical point of view SE can be differentiated by the presence or absence of prominent motor symptoms and by the degree of impaired consciousness (1). Focal status epilepticus (FSE) refers to focal epileptic seizures that last or recur over a period of >30 min. Both age and comorbidity are considered significant risk and unfavourable prognostic factors for SE (2).
Intravenous benzodiazepines are considered first-line treatment, while anti-epileptic drugs (AEDs) such as phenytoin (PHT), valproic acid (VPA), levetiracetam (LEV) and lacosamide (LCM) are the second-line agents of choice.
LCM is a relatively new AED which acts enhancing the slow inactivation of voltage-gated sodium channels. It has good pharmacokinetic properties and its clinical efficacy, safety and tolerability are shown by numerous studies (3; 4). LCM is currently approved for adjunctive treatment of focal-onset and focal to bilateral tonic-clonic seizures and for monotherapy in focal epilepsy and its use in SE seems safe, effective and well tolerated (5). Further evidence seems to be warranted to determine its use in populations of comorbid and/or elderly patients.
Case report
An 80-year old woman was admitted to our inpatient department due to an acute onset of non-fluent aphasia and involuntary rhythmical muscular jerks with a right facio-brachial distribution.
She had an history of arterial hypertension, previous NSTEMI myocardial infarction (MI) with subsequent coronary artery bypass surgery, COPD, severe carotid artery disease, previous surgical removal of a laryngeal tumor, previous surgical intervention for bowel obstruction with resection and stoma positioning and previous neurosurgical intervention of left frontal meningioma removal with subsequent development of focal epilepsy. Seizures were mostly focal with impaired awareness and motor onset characterized by a tonic contraction of the contralateral arm with bilateral afinalistic movements and oro-buccal automatisms. Sometimes a focal to bilateral tonic-clonic semiology was reported. In order to prevent seizures the patient was taking oral LEV, at a dosage of 1000 mg BID, and oral VPA, at a dosage of 300 + 500 mg/day. She was also taking other medications such as antiplatelet, beta-blocker and lipid-lowering drug therapy.
Vital signs were normal, except for a temperature of 38°C, and the patient appeared alert and with a mild tachypnea. A neurological examination was performed and the patient showed a non-fluent aphasia with a relatively preserved comprehension and a right side hemiparesis with continuous rythmical facio-brachial jerks. ECG was normal. Laboratory findings showed creatinine levels of 1.20 mg/dl (N.V. 0.73-1.18) with an eGFR of 43.91 ml/min, azotemia levels of 70 mg/dl (N.V. 18-55) and valproic acid levels of 48 mcg/ml (N.V. 50-100). CT scan of the head was negative except for signs of previous left frontal meningioma surgery.
Charlson Comorbidity Score (CCS) was 8 whereas a Status Epilepticus Severity Score (STESS) of 2/6 points was calculated (6; 7). A chest radiograph showed a right basal pneumonia and empirical ceftriaxone therapy was started.
An intravenous bolus of diazepam 10 mg repeated after 10 minutes did not control seizures; then we started VPA I.V. administration with a loading dose of 1200 mg and a subsequent continuous intravenous infusion of 1600 mg/day. Because of dysphagia and CKD, LEV was reduced from an oral dose of 2000 mg/day to an intravenous dose of 1000 mg/day. Since the patient continued presenting speech difficulties and focal motor jerks, a rapid I.V. infusion of LCM 400 mg was started, followed by a maintenance dosage of 200 mg BID. After the first bolus the patient presented reversal of the speech disturbance and muscular contractions, without any modification of her ECG.
An EEG recording was then performed, showing focal slow waves mixed to interictal epileptiform discharges over the left fronto-temporal regions.
A CT scan of the thorax confirmed right lobe basal pneumonia and intravenous antibiotic therapy with piperacillin/tazobactam was then started.
The patient presented a good clinical response to therapies and at discharge she presented a normal neurological examination. Oral LEV was progressively stopped because of CKD, oral VPA was augmented to 1500 mg/day and oral LCM 200 mg BID was added to the chronic therapy. The patient was then discharged to home.
After 3 months the patient was seizure-free and EEG showed a dramatic reduction of epileptiform discharges.
Discussion
We herewith report the case of an FSE in a patient suffering from focal epilepsy with a structural etiology. She previously developed focal seizures after surgical removal of a left-frontal meningioma. Her medical history was also characterized by cardiovascular, respiratory and gastrointestinal diseases. At the time of presentation the patient also received a diagnosis of aspiration pneumonia and moderate chronic kidney disease (CKD) based on clinical and laboratory findings.
The patient presented a FSE with a main symptomatic remote etiology, although it is possible that additional factors like infection and drug underdosing due to poor compliance contributed to the clinical picture. All of these potential causes were predictors of good outcome (8).
STESS is a useful clinical tool to reliably assess in-hospital mortality of patients with SE. Although STESS of 2/6 points expressed a good short-term outcome the multiple medical comorbidity burden (CCS 8) associated with advanced age could significantly worsen the prognosis of our patient.
Figure 1.
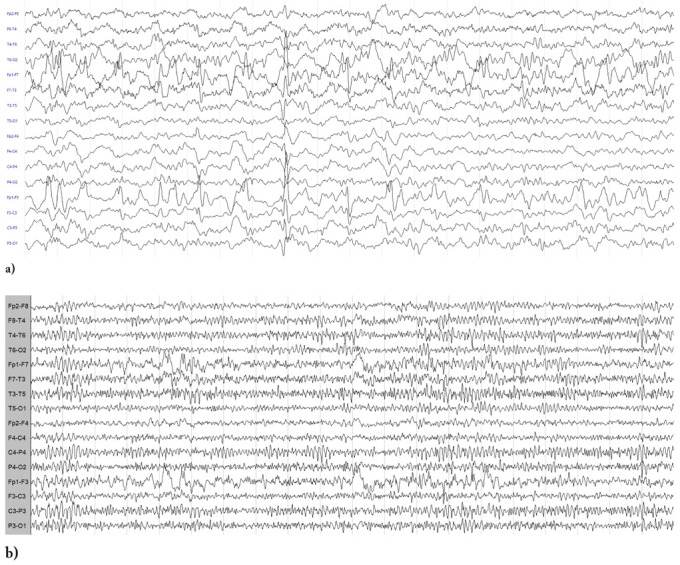
a) EEG showing slow and interictal epileptiform discharges over the left fronto-temporal regions; b) Control EEG at 3 months from hospital discharge showing a lesional slow activity over the left fronto-temporal regions
As the first-line AED failed to stop seizure activity, intravenous LCM treatment was started with complete reversal of the clinical picture (9). In a recent review, LCM proved its efficacy in terminating SE, particularly focal motor status epilepticus, and a good safety and tolerability profile was also reported (10). Anyway, reports regarding LCM treatment of SE in elderly are few (11).
In our case LCM proved to be efficacious in terminating seizures activity while remaining safe and tolerable even in a patient suffering from multiple medical conditions such as ischemic cardiomyopathy and moderate CKD. Moreover, we herewith confirm LCM good pharmacokinetic properties as we did not report any relevant drug-drug interaction, even while other intravenous drugs, such as antibiotics, were used.
Conclusion
Describing this case, we suggest that the use of LCM to treat SE might be an efficacious, safe and tolerable option even in the elderly patient with other comorbidities, although a prognostic evaluation needs to be done in order to always assure the best treatment benefit for the patient.
References
- 1.Lowenstein D. H, Rossetti A. O, Shinnar S, Trinka E, Shorvon S, Cock H, et al. A definition and classification of status epilepticus - Report of the ILAE Task Force on Classification of Status Epilepticus. Epilepsia. 2015;56(10):1515–1523. doi: 10.1111/epi.13121. [DOI] [PubMed] [Google Scholar]
- 2.Leppik I. E. Status epilepticus in the elderly. Epilepsia. 2018;59(March):140–143. doi: 10.1111/epi.14497. [DOI] [PubMed] [Google Scholar]
- 3.Fountain N. B, Krauss G, Isojarvi J, Dilley D, Doty P, Rudd G. D. Safety and tolerability of adjunctive lacosamide intravenous loading dose in lacosamide-naive patients with partial-onset seizures. Epilepsia. 2013;54(1):58–65. doi: 10.1111/j.1528-1167.2012.03543.x. [DOI] [PubMed] [Google Scholar]
- 4.Halász P, Kälviäinen R, Mazurkiewicz-Beldzin´ska M, Rosenow F, Doty P, Hebert D, Sullivan T. Adjunctive lacosamide for partial-onset seizures: Efficacy and safety results from a randomized controlled trial. Epilepsia. 2009;50(3):443–453. doi: 10.1111/j.1528-1167.2008.01951.x. [DOI] [PubMed] [Google Scholar]
- 5.Baulac M, Rosenow F, Toledo M, Terada K, Li T, De Backer M, et al. Efficacy, safety, and tolerability of lacosamide monotherapy versus controlled-release carbamazepine in patients with newly diagnosed epilepsy: a phase 3, randomised, double-blind, non-inferiority trial. The Lancet Neurology. 2017;16(1):43. doi: 10.1016/S1474-4422(16)30292-7. [DOI] [PubMed] [Google Scholar]
- 6.Alvarez V, Januel J. M, Burnand B, Rossetti A. O. Role of comorbidities in outcome prediction after status epilepticus. Epilepsia. 2012;53(5):89–92. doi: 10.1111/j.1528-1167.2012.03451.x. [DOI] [PubMed] [Google Scholar]
- 7.Aukland P, Lando M, Vilholm O, Christiansen E. B, Beier C. P. Predictive value of the Status Epilepticus Severity Score (STESS) and its components for long-term survival. BMC Neurology. 2016;16(1) doi: 10.1186/s12883-016-0730-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rossetti A. O, Hurwitz S, Logroscino G, Bromfield E. B. Prognosis of status epilepticus: Role of aetiology, age, and consciousness impairment at presentation. Journal of Neurology, Neurosurgery and Psychiatry. 2006;77(5):611–615. doi: 10.1136/jnnp.2005.080887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bauer S, Willems L. M, Paule E, Petschow C, Zöllner J. P, Rosenow F, Strzelczyk A. The efficacy of lacosamide as monotherapy and adjunctive therapy in focal epilepsy and its use in status epilepticus: Clinical trial evidence and experience. Therapeutic Advances in Neurological Disorders. 2017;10(2):103–126. doi: 10.1177/1756285616675777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Strzelczyk A, Zöllner J. P, Willems L. M, Jost J, Paule E, Schubert-Bast S, et al. Lacosamide in status epilepticus: Systematic review of current evidence. Epilepsia. 2017;58(6):933–950. doi: 10.1111/epi.13716. [DOI] [PubMed] [Google Scholar]
- 11.Rainesalo S, Mäkinen J, Raitanen J, Peltola J. Clinical management of elderly patients with epilepsy; the use of lacosamide in a single center setting. Epilepsy and Behavior. 2017;75:86–89. doi: 10.1016/j.yebeh.2017.07.045. [DOI] [PubMed] [Google Scholar]


