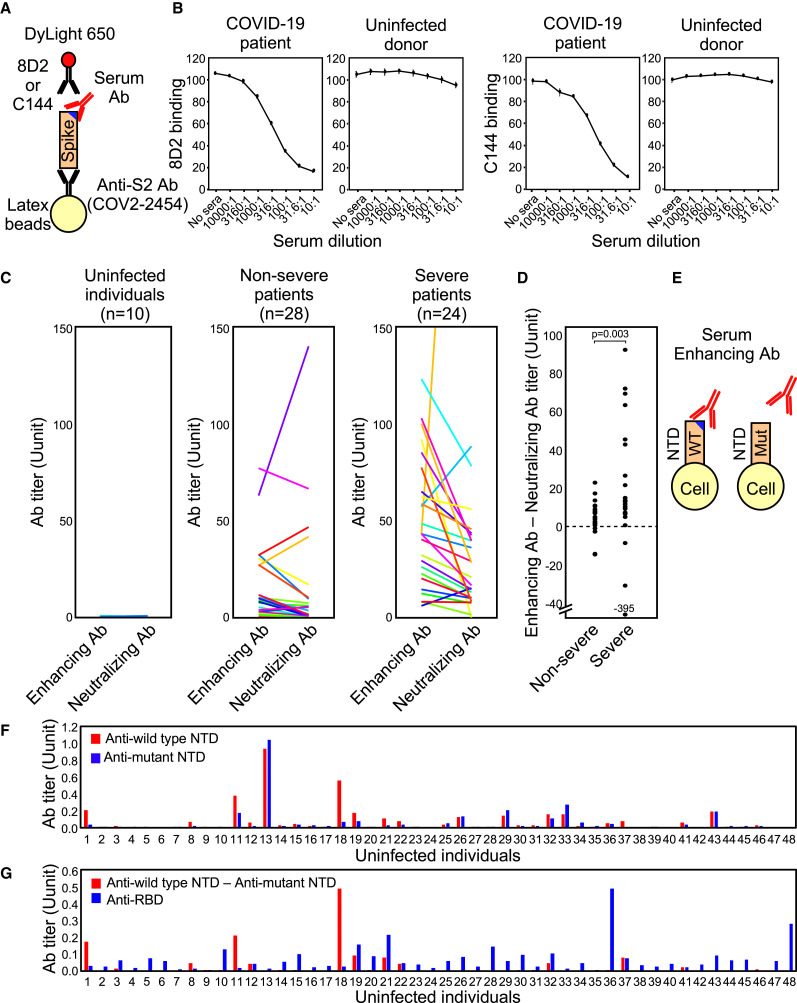
Figure 7.
SARS-CoV-2 infectivity-enhancing antibodies in COVID-19 patients and uninfected individuals
(A) A method of detecting the enhancing or neutralizing antibodies using a competitive binding assay. DyLight-650-labeled 8D2 and C144 were used to detect the enhancing and neutralizing antibodies, respectively.
(B) The binding of the enhancing antibody, 8D2, or the neutralizing antibody, C144, to beads coated with the spike protein was analyzed in the presence of the serially diluted serum of a representative COVID-19 patient or an uninfected donor.
(C) The levels of SARS-CoV-2 infectivity-enhancing antibodies and neutralizing antibodies in noninfected individuals and non-severe and severe COVID-19 patients.
(D) Enhancing antibody titers subtracted with neutralizing antibody titers were compared between non-severe and severe COVID-19 patients. Statistical significance derived from Mann-Whitney U test is indicated.
(E) Enhancing antibodies were detected by comparing antibody binding to the wild-type NTD-TM (WT) and the mutant NTD-TM lacking the enhancing antibody epitopes (Mut).
(F) Serum levels of antibodies in uninfected individuals against the wild-type NTD (blue bar) and mutant NTDs whose epitopes for the enhancing antibodies were mutated (red bar).
(G) SARS-CoV-2 infectivity-enhancing antibody titers were calculated by subtracting antibody levels against the mutant NTD from those against the wild-type NTD in uninfected individuals (red bar). Anti-RBD antibody titers were analyzed using RBD-TM transfectants (blue bar).
See also Figure S6.