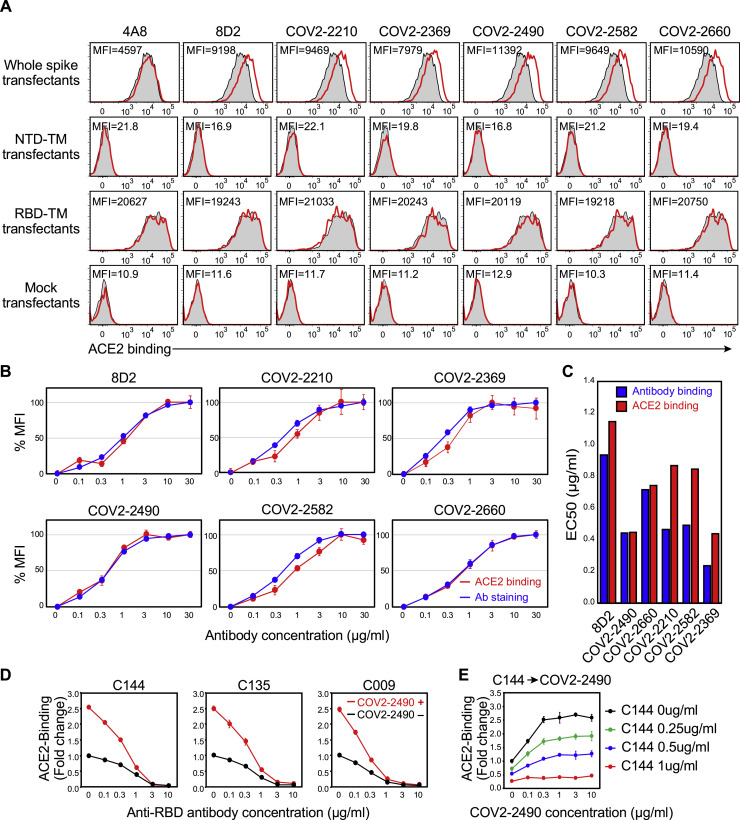
Figure S2.
Enhanced binding of ACE2 to the spike protein by specific anti-NTD antibodies, related to Figures 1 and 2
(A) The plasmids expressing the full-length spike, NTD-TM, RBD-TM, and mock were transfected into HEK293T cells with the GFP vector, and the transfectants were mixed with the indicated anti-NTD antibodies at 10 μg/ml. 4A8 is a non-enhancing antibody and the remaining is enhancing antibodies. Transfectants not mixed with antibodies were used as a control (shaded histogram). Afterward, the cells were stained with biotin-labeled ACE2-Fc fusion protein, followed by APC-labeled streptavidin. The fluorescence intensities of APC on the GFP-expressing cells are shown (red line). Mean fluorescent intensities (MFI) of red lines were shown in the figure.
(B) HEK293T cells transfected with spike protein were mixed with the indicated concentrations of enhancing antibodies. Subsequently, APC-conjugated anti-human IgG Fc antibody (blue) or biotin-ACE2-Fc fusion protein, followed by APC-labeled streptavidin (red), were mixed with the transfectants. The mean fluorescence intensities of cells stained in the absence of the enhancing antibody were set to 0, and the maximum fluorescence intensities in the presence of the enhancing antibody were set to 100. EC50 was calculated from the concentrations of the enhancing antibody that induced half the maximum fluorescence intensities.
(C) EC50s of antibody binding to the spike transfectants and ACE2 binding enhancement by the antibodies were shown.
(D) ACE2-Fc binding to wild-type spike protein in the presence of COV2-2490 antibodies at 3 μg/ml and various concentrations of anti-RBD neutralizing antibodies indicated in the figure (red line). ACE2-Fc binding in the absence of the enhancing antibodies was shown as the control (black line).
(E) Neutralizing C144 Ab was added to the spike transfectants first and then an enhancing antibody COV2-2490 was added. Concentrations of antibodies were indicated in the figure. ACE2-Fc binding to wild-type spike protein was shown. The data are presented as mean ± SD. The representative data from three independent experiments are shown.