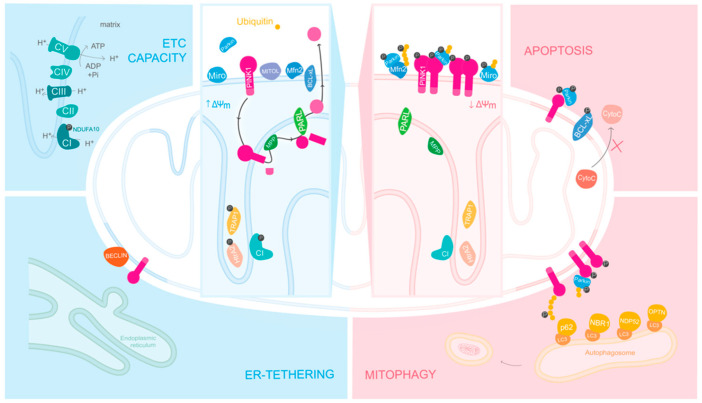
Figure 2.
PINK1 has different roles depending on mitochondria’s overall state. In the presence of healthy mitochondria, PINK1 is internalized and phosphorylates, among other substrates, the complex I subunit NDUFA10 at the inner mitochondrial membrane (IMM). By regulating the enzymatic activity of complex I, PINK1 modulates the overall electron transport chain (ETC) capacity and, ultimately, the overall output levels of ATP. Afterwards, PINK1 is sequential cleaved by the proteases MPP and PARL and released to the cytosol for degradation. PINK1 also has a protective role, as it phosphorylates Bcl-xl in order to inhibit apoptosis. When mitochondria are depolarized, PINK1 accumulates on the outer mitochondrial membrane (OMM), where it forms homodimers and undergoes autophosphorylation. After this, PINK1 recruits and phosphorylates Parkin, and consequently also phosphorylates ubiquitin. Due to Parkin’s E3 ubiquitin ligase activity, Parkin and PINK1 create a positive feedback-loop, recruiting more ubiquitin and Parkin to be phosphorylated, creating poly-ubiquitin chains all around the surface of damaged mitochondria. This targets mitochondria for degradation via mitophagy. Posteriorly, due to the recruitment of autophagic receptors, like LC3, OPTN and NDP52, damaged mitochondria are engulfed and degraded via autophagy. Upon depolarization, ER-tethering to mitochondria is also hampered as PINK1 accumulates on MAM structures recruiting Beclin1 to form omegasomes, which are autophagosome precursors. ΔΨm, mitochondrial membrane potential; CI, Complex I; CII, Complex II; CIII, Complex III; CIV, Complex IV; CV, Complex V or ATP synthase; P, phosphorylation.