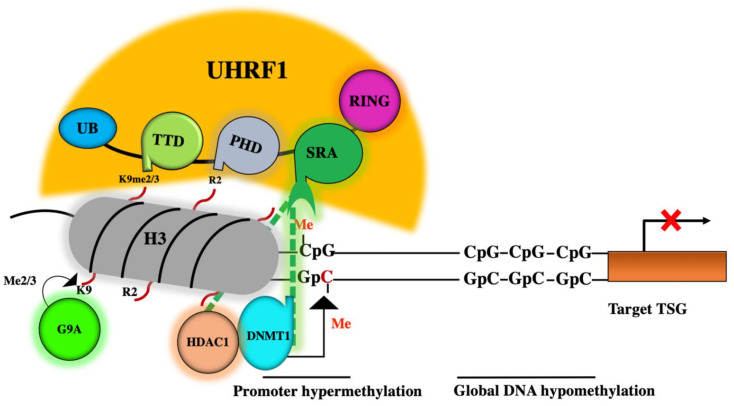
Figure 1.
Role of the epigenetic reader UHRF1 (ubiquitin-like containing plant homeodomain (PHD) and interesting new gene (RING) finger domains 1) in epigenetic silencing of tumor suppressor genes (TSGs). During DNA replication, the SET and RING-associated (SRA) domain of UHRF1 can read methylated CpG sites (hemimethylated DNA) located with TSG promoter. Via the SRA domain, UHRF1 also recruits DNA methyltransferase 1 (DNMT1) and guides it to methylate the unmethylated cytosine of the newly synthetized DNA strand, leading to hypermethylation of the TSG promoter with a global hypomethylation. Through the plant homeodomain (PHD) domain, UHRF1 can bind to unmodified arginine 2 of histone 3 and via its tandem Tudor domain (TTD) domain, UHRF1 can recognize and bind to di or trimethylation of lysine 9 of histone 3 (H3K9me2 or H3K9me3). UHRF1 also uses its SRA domain to recruit histone deacetylase 1 (HDAC1) and recruits histone methyltransferase G9a, leading to histone 3 deacetylation and methylation, respectively. The consequence is the epigenetic silencing of TSGs.