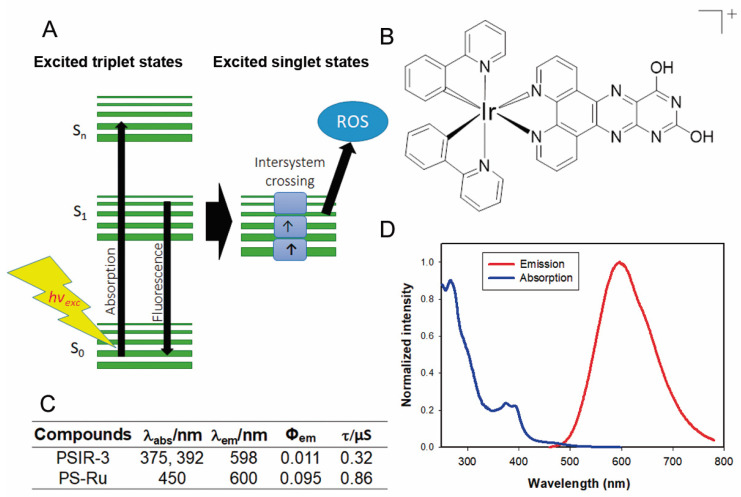
Figure 4.
The photophysical properties of the PSIR-3 photosensitizer. (A) schematic representation of the absorption–emission process of photosensitizer molecules. Light excites external electrons to accede from a basal state S0 to a higher energetic state S1–Sn. Suppose the electrons return to its ground state; the energy is then released as fluorescence. However, when the excited electron enters an intersystem crossing process, the released energy excites molecular oxygen and converts it to reactive oxygen species (ROS). (B) chemical structure of the Ir(III) compound (PSIR-3, [Ir(ppy)2(ppdh)]PF6). (C) Summary of the photophysical properties of the PSIR-3 and PS-Ru compounds, where λabs = wavelength of absorbance, λem = wavelength of emission, Φem = emission quantum yield, and τ = time in the excited state. Data for PS-Ru were obtained from the literature. Adapted from [42], Elsevier, 2010; Adapted from [53], American Chemical Society, 1983. (D) The absorption and the emission spectra of the PSIR-3 in acetonitrile (ACN).