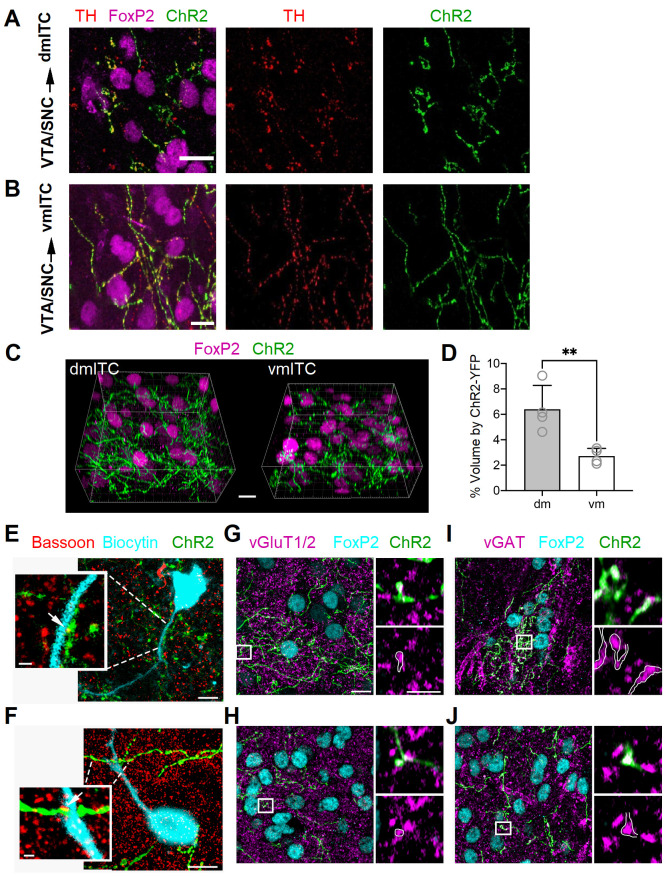
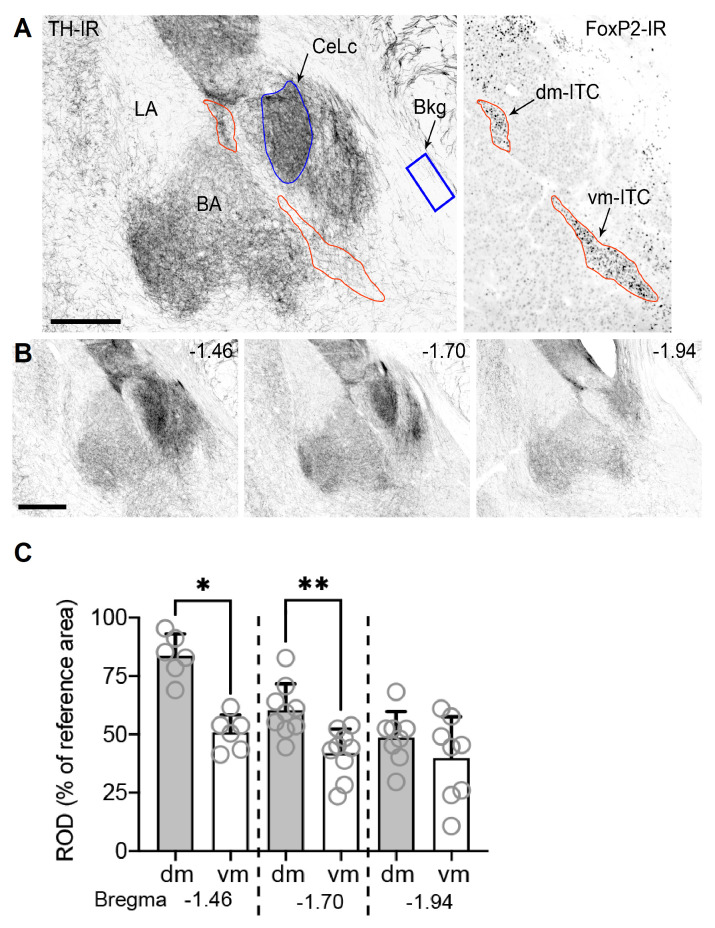
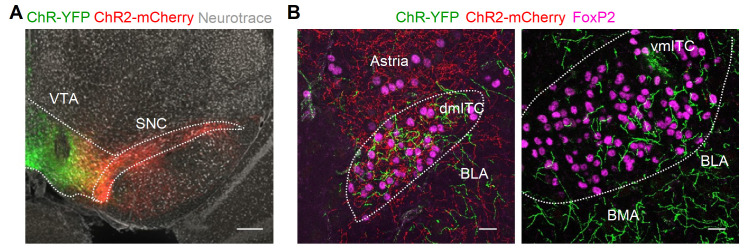
Figure 1. Dopaminergic fibers from VTA/SNC targeting dm- and vm-ITC clusters in the amygdala co-label with vGAT and vGluT1/2.
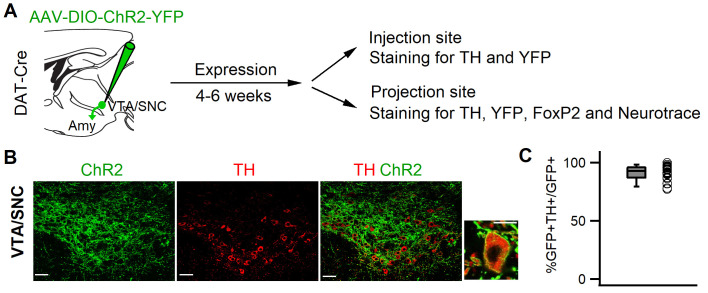
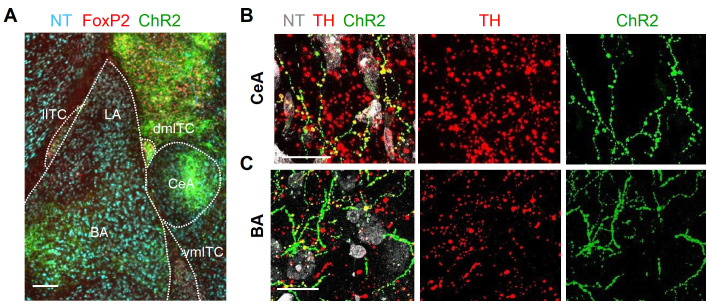
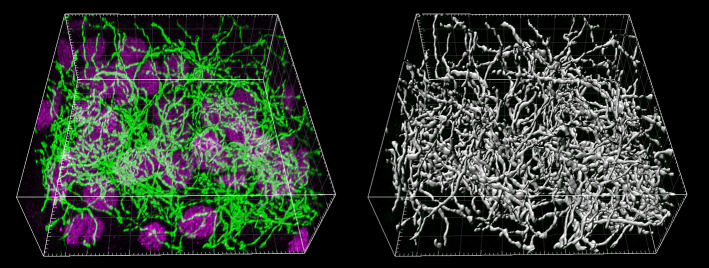
(A–B) Maximum intensity projection confocal images illustrating ChR2-YFP+ axons (green) originating from tyrosine hydroxylase (TH)-positive dopaminergic neurons (TH, red) in the ventral tegmental area/substantia nigra pars compacta (VTA/SNC) targeting FoxP2-positive neurons (magenta) in the dorsomedial (dm)- and ventromedial (vm)-intercalated cell (ITC) clusters. Overlay (left), TH staining (middle), ChR2-YFP (right). Scale bars: 10 µm. (C) Representative image volumes of the dm- and vm-ITC clusters with FoxP2-labeled nuclei (magenta) and ChR2-YFP+ axons (green). Scale bar: 10 µm. (D) The volume fraction encompassed by ChR2-YFP+ axons was significantly higher in the dm-ITC (6.40 ± 0.94% of total image volume) compared to the vm-ITC cluster (2.72 ± 0.30% of total image volume; unpaired t-test, **p=0.0097). Histograms display the mean ± SEM and values from individual mice (empty circles, n=4 animals). The average dm- and vm-ITC cluster volume analyzed per animal was 138.15 ± 29.51 and 156.12 ± 10.29 µm3, respectively. (E–F) Overlay confocal images of ChR2-YFP+ axons (green) and biocytin-filled cells (turquoise) in dm-ITC and vm-ITC clusters immunostained for the presynaptic marker Bassoon to examine putative active zones (white arrows). Scale bars 5 and 1 µm (inset). (G–H) Confocal images of dm- and vm-ITC clusters, including FoxP2-positive neurons (turquoise) that contain ChR2-YFP+ fibers (green) co-labeled for the presynaptic markers vGluT1/vGluT2 (magenta). Right panels show a higher magnification of the boxed area containing an example of a bouton co-expressing ChR2-YFP and vGluT1/vGluT2 outlined in white. (I–J) Confocal images of dm- and vm-ITC clusters, including FoxP2-positive neurons (turquoise) that contain ChR2-YFP fibers (green) co-labeled for the presynaptic marker vGAT (magenta). Right panels show a higher magnification of the boxed area containing an example of a bouton co-expressing ChR2-YFP and vGAT outlined in white. Scale bars for (G–J): 10 µm for left panels, 3 µm for right panels. Thickness of confocal z-stacks: (A) 11.1 μM; (B) 12.5 μM; (E) 8.06 and 2.01 µm for the cell and synapse on dendrite, respectively; (F) 12.2 and 2.2 µm for the cell and synapse on dendrite, respectively; (G–J) left panels, 8.83 µm; right panels, single plane of 0.18 µm nominal thickness.