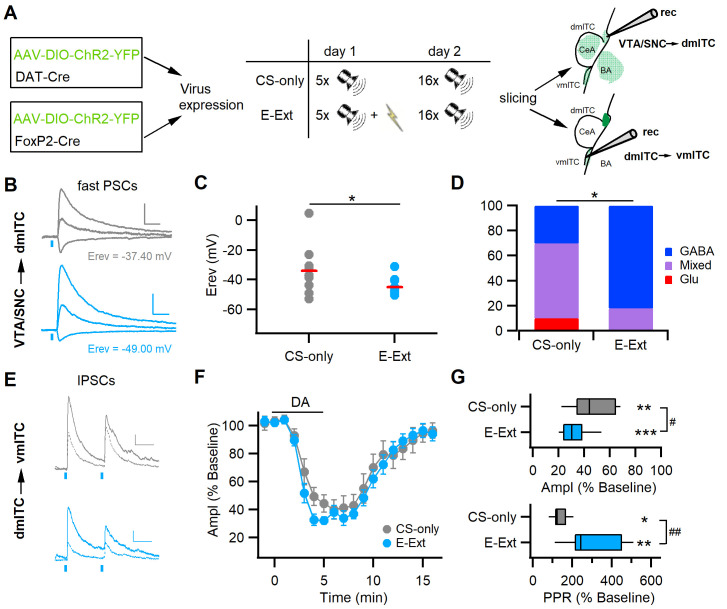
Figure 6. Early extinction enhances GABA release from midbrain terminals and DA-mediated depression of the dm-ITC→vm-ITC pathway.
(A) Experimental scheme: To investigate the VTA/SNC→dm-ITC pathway, ChR2 was transduced in dopaminergic midbrain neurons of DAT-Cre mice. To investigate dopamine (DA) modulation of the dm-ITC→vm-ITC pathway, ChR2 was transduced into the dorsomedial-intercalated cell (dm-ITC) cluster of FoxP2-Cre mice. The early extinction group (E-Ext) underwent fear conditioning on day 1 (5 conditioned stimulus-unconditioned stimulus (CS-US) pairings) and early extinction training on day 2 (16 CS presentations). The CS-only group received only CS presentations. (B) Left: Example traces of light-evoked postsynaptic currents (PSCs) by dopaminergic fiber stimulation recorded in dm-ITCs at −70, 0, and 40 mV from CS-only (grey traces) or E-Ext animals (blue traces). Scale bars: 50 pA, 50 ms. (C) Plot of PSC reversal potentials in individual dm-ITCs (dots) and average (red lines) from CS-only and E-Ext groups. Erev was significantly lower in the E-Ext (−45.09 ± 1.76 mV, n=11 cells from six animals) vs. the CS-only group (−34.06 ± 5.11 mV, n=10 cells from four animals, *p=0.047, paired t-test). (D) Summary graph comparing the type of fast PSCs in dm-ITCs recorded from CS-only and E-Ext animals (CS-only, n=10: GABA 30%, mixed 60%, Glu 10%; E-Ext, n=11: GABA 82%, mixed 18%). PSC types were significantly different between groups (Fisher’s exact test = 5.68, *p=0.041). (E) Example traces of light-evoked inhibitory postsynaptic currents (IPSCs) recorded in a ventromedial-intercalated cell (vm-ITC) at 0 mV upon paired pulse stimulation (100 ms interstimulus interval) of the dm-ITC→vm-ITC pathway from CS-only (grey traces) or E-Ext (blue traces) animals before (solid) and during DA application (dotted). Scale bars 50 pA, 50 ms. (F) Time course of changes in IPSC amplitude upon bath application of DA (30 µM, 5 min) in dm-ITC→vm-ITC pathway in CS-only and E-Ext groups. Two-way ANOVA (1–9 min) revealed significant changes for time, F(8)=37.903, p<0.001, and group, F(1)=8.229, p=0.005, but no significant interaction, F(8, 144)=0.521, p=0.839. (G) Significant changes of IPSC amplitude (paired t-tests: CS-only, **p=0.002; E-Ext, ***p<0.001) and paired pulse ratio (PPR) (paired t-tests: CS-only, *p=0.040; E-Ext, **p=0.003) 4–5 min after DA application in both groups. IPSC amplitude was more depressed in neurons recorded from E-Ext vs. CS-only animals (32.42 ± 3.16%, n=11 cells from six animals, vs. 46.75 ± 6.14%, n=7 cells from four animals, unpaired t-test, #p=0.036). The PPR increase was larger in neurons recorded from E-Ext vs. CS-only animals (310.63 ± 42.64%, n=11, vs. 139.68 ± 15.43%, n=7, unpaired t-test, ##p=0.007).