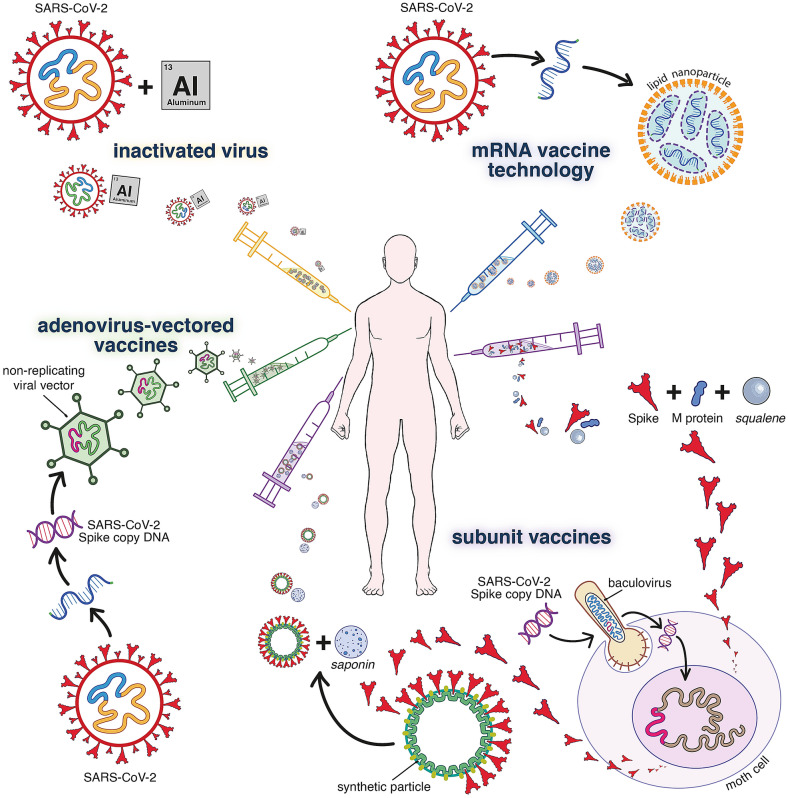
Fig. 1.
Technologies on which the main current COVID-19 vaccines or vaccine candidates are based. Inactivated vaccines such as Sinovac (CoronaVac®), Sinopharm (BBIBP-CorV®) or Bharat Biotech (BBV152/Covaxin®) are produced with traditional methods (similar to rabies or hepatitis A vaccines), where the SARS-CoV-2 virus is grown in cell culture, inactivated by β-propiolactone and adsorbed to alum (and other excipients). Current S-based vaccines consist of either (i) recombinant S protein (like hepatitis B vaccines), (ii) replication-deficient viral vectors expressing S, or (iii) mRNA encoding S. Because of an optimized design, these vaccines induce rapid and vigorous immune responses. For the subunit vaccines, recombinant S is produced in insect cells and formulated with saponin or other emulsions as adjuvant. In the case of NVX-CoV2373 by Novavax, S subunits are mounted on a synthetic protein-scaffold to further enhance their immunogenicity. The vector-based vaccines by AstraZeneca (AZD1222/Covishield®), Gamalenya (Gam-COVID-Vac/Sputnik V®) or Johnson & Johnson (Ad26.COV2.S/JNJ-78436735) use modified replication-deficient adenoviruses (respectively chimpanzee AdV; a combination of AdV 26 and AdV5; Adv26) to deliver an expression cassette for the copy DNA encoding for S (alike established e.g. for gene therapy). For the novel mRNA approach, synthetic mRNA encoding for S is encapsulated in lipid nanoparticles. Representative mRNA vaccines are BNT162b2 (Comirnaty®) by Pfizer/BioNtech, mRNA-1273 by Moderna and CVnCoV by Curevax. All current COVID-19 vaccines are administered by intramuscular injection; following a two-dose regimen (with as exception Ad26.COV2.S as single dose). For further information, see Kyriakidis et al. NPJ Vaccines. (2021) https://doi.org/10.1038/s41541-021-00292-w