Abstract
Background
Neural oscillations in the cerebral cortex are associated with a range of cognitive processes and neuropsychiatric disorders. However, non-invasively modulating oscillatory activity remains technically challenging, due to limited strength, duration, or non-synchronization of stimulation waveforms with endogenous rhythms.
Objective
We hypothesized that applying controllable phase-synchronized repetitive transcranial magnetic stimulation pulses (rTMS) with alternating currents (tACS) may induce and stabilize neuro-oscillatory resting-state activity at targeted frequencies.
Methods
Using a novel circuit to precisely synchronize rTMS pulses with phase of tACS, we empirically tested whether combined, 10-Hz prefrontal bilateral stimulation could induce and stabilize 10-Hz oscillations in the bilateral prefrontal cortex (PFC). 25 healthy participants took part in a repeated-measures design. Whole-brain resting-state EEG in eyes-open (EO) and eyes-closed (EC) was recorded before (baseline), immediately (1-min), and 15- and 30-min after stimulation. Bilateral, phase-synchronized rTMS aligned to the positive tACS peak was compared with rTMS at tACS trough, with bilateral tACS or rTMS on its own, and to sham.
Results
10-Hz resting-state PFC power increased significantly with peak-synchronized rTMS + tACS (EO: 44.64%, EC: 46.30%, p < 0.05) compared to each stimulation protocol on its own, and sham, with effects spanning between prefrontal and parietal regions and sustaining throughout 30-min. No effects were observed with the sham protocol. Moreover, rTMS timed to the negative tACS trough did not induce local or global changes in oscillations.
Conclusion
Phase-synchronizing rTMS with tACS may be a viable approach for inducing and stabilizing neuro-oscillatory activity, particularly in scenarios where endogenous oscillatory tone is attenuated, such as disorders of consciousness or major depression.
Keywords: Transcranial magnetic stimulation, Transcranial alternating current stimulation, Neuromodulation, Oscillations, Electroencephalography, Prefrontal cortex, Alpha, EEG, TMS, tACS
Highlights
-
•
Non-invasively inducing and stabilizing neural oscillations remains challenging.
-
•
We develop a controllable phase-synchronized circuit to combine rTMS and tACS.
-
•
This circuit was tested for inducing 10 Hz oscillations in healthy prefrontal cortex.
-
•
10 Hz rTMS synchronized to the positive 10 Hz tACS peak induced stable after-effects.
-
•
Phase-synchronized stimulation is a viable approach for oscillatory neuromodulation.
Introduction
A critical neuroscientific objective in recent years has been towards advancing the development of techniques that can interface with complex neurobiological systems such as the human brain, in order to understand how salient physiological properties, such as neural oscillations, underlie cognition and behaviour. Although non-invasive neuroimaging techniques such as electro/magnetoencephalography (EEG/MEG) have provided substantial correlational evidence linking cognitive processes with oscillatory signatures, there remains a lagging number of interventional studies for demonstrating their causality.
In this regard, non-invasive brain stimulation methods such as transcranial magnetic and electric stimulation (TMS, tES) have received increasing attention, due to their ability to alter neuronal oscillations at the system level [1], while also showing treatment efficacy in clinical settings, where aberrant oscillatory activity has been reported across a variety of neuropsychiatric disorders [2]. Specifically, two techniques have emerged over the past decade in order to meet these objectives, each delivering periodic electromagnetic waveforms non-invasively across the scalp: repetitive transcranial magnetic stimulation (rTMS), which induces transient frequency-specific modulation through strong supra-threshold electromagnetic pulses, which however resets within minutes following the end of stimulation [3], and low-intensity transcranial alternating current stimulation (tACS), which has been shown to result in lasting elevation/alteration of oscillatory activity but only in frequency ranges already present at a relevant level endogenously [[4], [5], [6]]. However, in scenarios where endogenous oscillatory activity is minor or absent, e.g. high frequency oscillations in patients with disorders of consciousness [7]—or aberrant, e.g. in epilepsy [8], these techniques appear to be limited for safely achieving lasting results. Evidence obtained by direct recordings in animal experiments suggests that tACS synchronization of oscillation frequencies that are weaker or deviate more from endogenous rhythms may require increasing the stimulation strength respectively [9,10]. At the same time, a critical issue facing the field at large is confounding effects of inter-individual variability, including from both rTMS [[11], [12], [13]] and tACS [14,15]. Presumably, some of this variability may be due to insufficient electric fields reaching the targeted region, as shown by a recent tACS-MEG study showing that the between-subject variability of tACS outcomes could be linked to inter-individual anatomical differences in the electric field [16]. As such, devising novel or optimizing current neuromodulation protocols which can more effectively target and engage brain oscillations may help to address these aforementioned challenges.
Given that the presently identified mechanisms of rTMS and tACS can be characterized by unique, yet reciprocal modes of affecting neurophysiological activity and plasticity, a few recent studies have explored the concept of combining rTMS with tACS/oscillatory tDCS (o-tDCS). Low-frequency (0.8 Hz) o-tDCS combined with continuous theta burst stimulation (cTBS) was found to be efficient in enhancing the neuroplastic after-effects of cTBS on reduction of cortical excitability [17]. Similarly, cTBS applied in-phase with the trough of 10-Hz tACS [18] and intermittent theta burst stimulation (iTBS) applied simultaneously with 70 Hz tACS [19] was found to be efficient in enhancing respective neuroplastic after-effects, as indexed by changes in motor cortical excitability. However, an open question is whether combined and phase-synchronized rTMS and tACS may lead to an induction and long-lasting stabilization of oscillations in areas with low oscillatory tone of the target frequency, which could arise via neural entrainment [[20], [21], [22]] and/or plasticity [4,23,24].
Here, this question was empirically explored. First, an electrical optocoupler-based circuit was developed in-house (Figure S1), which allowed us to achieve precise, bidirectional synchronization between rTMS and tACS, using industry-standard commercial stimulator devices (a TMS device equipped with a BNC-based TTL input trigger and an 8-channel tACS stimulator). This circuit was then evaluated in a series of experiments where stimulation was applied at 10 Hz simultaneously and bilaterally over the dorsolateral prefrontal cortex--a region with low endogenous alpha activity during wakefulness, but a crucial hub region for performing network modulation, given its functional role in many cognitive processes [25].
Materials and methods
Ethical approval
The experiment conformed to the principles of the Declaration of Helsinki and was approved by the Institutional Review Board (Ethics Committee) of the Leibniz Research Center for Working Environment and Human Factors (Dortmund, Germany). The datasets generated during the current study are available from the corresponding author on reasonable request.
Subjects
25 healthy individuals were recruited (13 females, mean age 26.36, SD 3.85, age range 19–33 years; Table S1). Primary criteria for exclusion were age less than 18 or greater than 50 years, smokers, pregnant, history of neurological or psychiatric disorders, including epilepsy or head trauma, physical impairments affecting the usage of both hands, and having metal implants. Prior to the beginning of experimental sessions, all participants were screened by a medical professional to verify a healthy neurological history. All subjects provided their written informed consent and were compensated for participation.
Stimulation
We developed a custom circuit to synchronize pulses of transcranial magnetic stimulation (TMS) at precise phases within the waveform of transcranial alternating current stimulation (tACS). Fig. 1A presents an overview of the circuit (see Figure S1 for a detailed schematic). With regard to TMS, pulses were delivered using two figure-of-eight shaped coils (double 70 mm, PMD coil type; Mag and More GmbH, Munich, Germany). For sham TMS conditions, two separate sham coils were used (double 70 mm pCool-SHAM coil) which elicited audible clicks at the same corresponding decibel intensity as an active/real coil, but without inducing electromagnetic pulses. In order to deliver TMS pulses through two coils with minimal latency between pulses, the TMS devices were connected in series with TTL signals triggering the TMS. Timing between the two pulses was verified to have near-zero latency through measurements obtained from an oscilloscope. With regard to tACS, we used a Starstim 8-channel constant-current, battery-powered electric stimulator (Neuroelectrics, Barcelona, Spain). Stimulation electrodes were circular (2 cm radius, 12.57 cm2 area) and made of carbon rubber, with a connector position located directly at the center. Conductive Ten20 paste (Weaver and Company, USA) was applied to the bottom of the electrodes. Care was taken to ensure the electrodes maintained full contact with the scalp, with hair moved to the side, and mesh straps used to ensure positional alignment. The multichannel Starstim device was customized to allow external programming of custom stimulation waveforms at fixed latencies and current amplitudes through the 8 available channels. As such, two channels were used to program square pulses at precise timepoints for delivering rTMS pulses in relation to four other channels that were used to deliver sinusoidal alternating current delivered to the head (see Figure S1). Square pulses (0.1 mA peak, 0.5% duty cycle) were delivered from the electric stimulator to a separate circuit containing an opto-coupler (developed in-house) in order to shield the stimulation from the main power. Current-to-voltage conversion resulted in a 5 V signal which was used as a TTL signal to communicate with the TRIGGER-IN on the TMS machine. Latencies in the waveform generated by the electric stimulator and the TMS stimulator were measured and verified to be in alignment (+/− 1 ms resolution) through means of an oscilloscope.
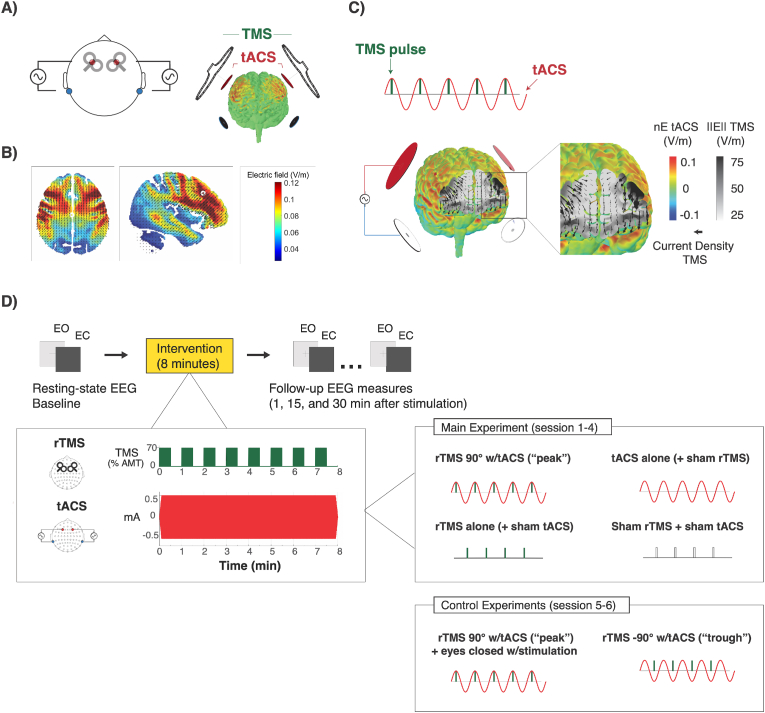
Fig. 1.
Computational modeling of methodology and experimental design. A) The multi-modal rTMS and tACS stimulation montage consisted of four tACS electrodes from a multichannel stimulator, with two electrodes applying sinusoidal current in the same phase over F3 and F4 EEG positions, and the other two applying currents in the opposite phase over the left and right mastoid positions (TP9 and TP10). TMS coils were positioned at an angle of 45° to the midline over each target tACS electrode during stimulation. B) Electric field simulation displaying the electric field magnitude induced by tACS. C) Electric field simulation displaying the normal component of the tACS electric field on the gray matter surface at the tACS peak when rTMS pulses were delivered in the prefrontal cortex (SimNIBS, v3.0). The color gradient represents the magnitude of the normal component of the electric field (inward normal convention, with positive values reflecting orthodromic direction with excitatory perturbation). Arrows indicate the direction of the TMS electric field, and the greyscale represents its magnitude inside the gray and white matter volume. D) Experimental design: sessions began with a baseline recording of resting-state EEG in eyes open (EO) and eyes closed (EC) conditions for 2 min each. Stimulation was applied for 8 min: rTMS was applied in 30 s on/off intervals at 70% of the participant’s active motor threshold intensity. tACS was applied continuously for 8 min, at 1 mA peak-to-peak intensity between each pair of tACS electrodes. The stimulation protocols applied are shown under each experiment’s heading, with green bars representing TMS pulses and red waveforms representing a single frontal tACS channel (identical with the bilateral frontal channel). Note that the waveform of the return electrodes (not shown here) were in opposite phase to the frontal electrodes. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
The final multimodal and multichannel stimulation montage consisted of four tACS electrodes, with two channels delivering alternating current in the same phase from the left and right prefrontal cortex to the respective ipsilateral mastoid region (Fig. 1A), which resulted in an electric field distribution spanning from dorsal to deeper ventral regions of the frontal cortex, including also portions of the thalamus, according to our finite-element modeling (Fig. 1B). Stimulation electrodes of one polarity were positioned at the F3 and F4 EEG positions, while electrodes of the opposite polarity were positioned over the TP9 and TP10 positions. The respective electrodes from the EEG cap (EasyCap GmbH, Germany), as well as the four adjacent electrodes on each side were removed in order to prevent stimulation current shunting into the EEG electrodes. Two TMS coils were positioned directly over each prefrontal tACS electrode, and received signals from the tACS device through our circuit which triggered pulses at precise timepoints relative to the tACS sinusoid (Figure S1). Electric field modeling confirmed that the induced electric field excitatory effects from both TMS and tACS were aligned and maximal when the TMS pulses arrived at the “peak” of the tACS-induced sinusoids (Fig. 1C).
Electroencephalography
EEG was recorded using a 64-channel EEG system (NeuroOne, Mega Electronics Ltd., Finland). In order to avoid interference or saturation of the EEG amplifier during recording and stimulation, we measured neural activity from 52 Ag/AgCl EEG electrodes, excluding 10 channels that were underneath or adjacent to the stimulation electrodes (center points on F3, F4, TP9 and TP10). Additionally, two channels recorded left and right eye movements, a respiration belt over the chest recorded breathing, and bipolar electrodes over the left clavicle and lower rib cage recorded ECG. The sampling frequency was 2000 Hz, and EEG impedance maintained values less than 5 kOhms. During recording, digital TTL triggers transmitted to the EEG system by a presentation PC were used to demarcate the start and end of all experimental conditions.
Overall experimental procedures
Each participant attended all experimental sessions at the same time of day (either 9am, 12pm, or 3pm) to control for daily differences in arousal, with sessions separated by at least seven days to avoid carry-over effects. The experimental laboratory was illuminated with artificial lighting, and sound-proofed, and participants were additionally prepared with sound-attenuating headphones. Participants were seated comfortably in a reclined chair, with an adjustable vacuum pillow placed behind the neck to support and maintain the position of the head throughout the stimulation and recordings. In a preliminary session, each participant’s active motor threshold (AMT) was determined by applying single pulses of TMS over the left primary motor cortex in order to elicit motor evoked potentials from the right abductor digiti minimi (ADM) muscle. For each individual, 70% of the stimulation intensity required to produce the AMT was used as that participant’s rTMS intensity for the main experimental sessions. A 64-channel EEG cap was prepared on the participant’s head according to the 10-10 convention, and connected directly to the recording system along with additional channels for monitoring left/right EOG, bipolar ECG, and respiration. Baseline EEG recordings in eyes open (EO) and eyes closed (EC) conditions were recorded for 2 min each. During the EO condition, participants were instructed to fixate on a white cross presented on an LCD monitor (precisely 1 m away from the eyes in distance). Following the baseline recordings, stimulation was delivered for 8 min according to the randomized sequence of protocols (see below). In all sessions, tACS electrodes were prepared and a TMS coil (either active or sham) was used. With the exception of the control experiment with a stimulation protocol requiring eyes to be closed, participants were instructed to keep their eyes open and fixate on a cross presented on a monitor during the 8 min stimulation. Following the stimulation, 2 min eyes open and eyes closed resting state recordings were collected (timepoint “1 min”), and again after 15 min and 30 min from the end of the stimulation (Fig. 1D). Compliance to instructions was continuously monitored by a closed-circuit video camera.
Main experiment
The main experiment was designed to assess whether combined rTMS + tACS stimulation was more effective than each stimulation protocol on its own. We designed four stimulation protocols in a randomized sequence (right side of Fig. 1D): 1) rTMS at the peak of the tACS; 2) rTMS alone (with sham tACS); 3) tACS alone (with sham rTMS); 4) sham rTMS with sham tACS. Sham tACS consisted of a short fade-in and fade-out of stimulation over 6 s to mimic the cutaneous sensation of real tACS. The sham rTMS condition used a specialized TMS coil which applied artificial pulses of auditory stimuli at the same intensity as real TMS. Stimulation intensity and duration were chosen in a manner that were in compliance with technical and safety limits [26]: 10 Hz rTMS was applied at 70% of each individual’s active motor threshold in 30-sec on/off increments for 8 min (2400 total double pulses), while tACS was applied for 8 min continuously at 1 mA, with a 6 s fade-in/fade out at the beginning and end of stimulation. Two experimenters held and maintained the coil over the F3 and F4 stimulation sites, with the coil pointing 45° to the midline. Electrode cables were only connected during the course of the stimulation, and then detached for the remainder of the recording.
Control experiments
In addition to the main experiment, two additional control experiments with the following protocols were conducted: 5) rTMS at the positive peak of the tACS, with participants also instructed to maintain their eyes closed during stimulation (n = 25), and 6) rTMS at the negative trough of the tACS (n = 13). In order to deliver rTMS at the negative trough of the tACS (−90deg), the custom waveform protocol was modified to shift the alignment of the square waves to the trough of the sinusoidal current corresponding to the frontal F3 and F4 tACS channels.
Offline EEG analysis
As can be seen in the EEG power spectrogram (Fig. 2A), the overlaying rTMS and tACS stimulation artifact in the EEG prevented a straightforward analysis of the online effects of stimulation. Although some approaches to removing the tACS artifact have been proposed [21,27], they have not been able to satisfactorily address spurious non-linear artifacts which arise from physiological sources as well as from the stimulation and recording hardware [28,29]. Nevertheless, stimulation-induced changes in EEG power at the 10 Hz frequency band could be reliably obtained immediately following the end of stimulation. Offline analysis of EEG was performed using the Fieldtrip Toolbox in Matlab (R2018b). Raw data was down-sampled to 512 Hz. Independent component analysis (method ‘runica’) was used to remove eye-blink, respiration and cardiac artifacts. 50 Hz notch filtering was further applied to reduce power line noise. The remaining EEG data within the 2-min resting state EO and EC conditions were then epoched into 1 s trials, each with 500 ms overlap. Artefactual trials with channel variance exceeding three standard deviations from the mean were rejected and noisy channels with trial variance exceeding three standard deviations from the mean were interpolated using a spline function.
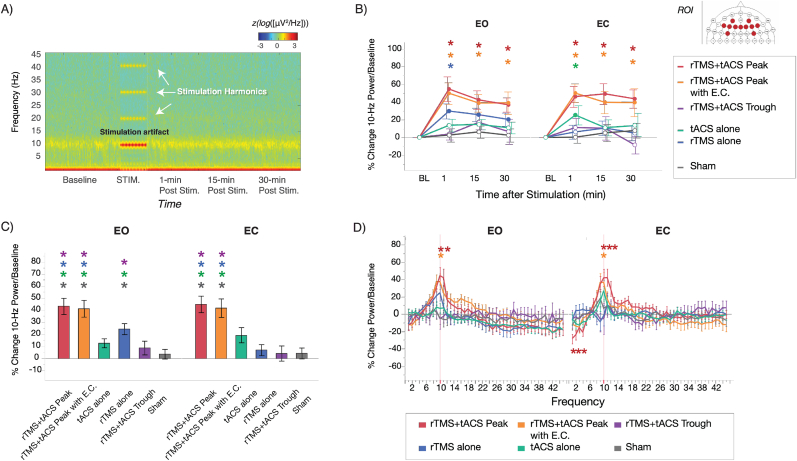
Fig. 2.
Results of experimental findings. A) Time-frequency spectrogram from the frontal Fz EEG channel during a representative recording session. The stimulation artifact as a result of both rTMS and tACS at 10 Hz can be seen during stimulation, which is accompanied by harmonic artifacts at 20, 30 and 40 Hz. These artifacts did not affect data quality through the remainder of the recording. B) Changes in 10 Hz EEG power from prefrontal electrodes (AF3-4, F1-6, Fz, FC3-4; see legend) as a function of stimulation protocol and time. Filled circles represent significant pair-wise differences relative to baseline, and asterisks represent significant pair-wise differences relative to sham (p < 0.05, corrected). Error bars indicate the standard error of means. During both, the eyes open (EO) and eyes closed (EC) resting state, 10 Hz power was enhanced relative to baseline and sham stimulation with the peak-synchronized rTMS + tACS protocol, as well as by the same protocol while participants maintained closed eyes during stimulation. Increased power was also observed immediately following stimulation with rTMS alone during EO and tACS alone during EC, which was significantly larger than sham only in the immediate 1-min post stimulation timepoint. C) Grand-average changes in 10 Hz power between pre- and post-stimulation timepoints (1–30 min). Asterisks, according to their color, indicate significant pairwise differences (p < 0.05, corrected). D) Overall, grand-average changes (1–30 min) in prefrontal activity across the power spectrum (1–45 Hz). The red line on 10 Hz indicates the targeted stimulation frequency. Asterisks indicate significant pairwise difference relative to sham (p < 0.05, corrected). (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Changes in power were statistically analyzed over the a priori pre-defined frequency (10 Hz), and space dimension (prefrontal F5, F6, Fz, FC3, FC4, AF3, AF4, F1, F2, F3 and F4 electrodes). Since electrode positions F3 and F4 were the sites of the tACS electrodes, activity from these channels was mathematically interpolated (spherical spline interpolation in Fieldtrip) using activity from adjacent channels (e.g. for the left prefrontal side (F3), activity from channels FC1, FC5, AF7, and AFz were used for interpolation). Pre-processed timeseries data were re-referenced to the common average of all EEG electrodes, and transformed to the spectral domain (1–45 Hz), using the multitaper fast-fourier transform (FFT) method in Fieldtrip (method ‘mtmfft’, Hanning tapers, 1 Hz frequency resolution). Power values for the ROI channels were extracted at the 10 Hz frequency bin, averaged together, and statistically analyzed (see Data Statistics).
In addition, a whole-brain analysis of resting state EEG was also conducted at the sensor level. Sensor level analyses were conducted in two levels. A first level analysis contrasted each participant’s session-wise change in 10 Hz activity relative to their pre-stimulation baseline. This analysis was conducted for each stimulation protocol and EO/EC conditions separately. Post-stimulation timepoints were averaged, and contrasted to the baseline map through a paired t-test analysis at the group level (two-tailed) using Fieldtrip’s ft_frequencystatistics function (method: “depSamplesT”). Resulting maps were adjusted for multiple comparisons using false-discovery rate (FDR) correction. In addition, a second-level analysis was conducted to contrast changes between stimulation protocols. As such, we first computed, for each participant and session, the baseline-normalized map of change in 10 Hz power between all post-stimulation timepoints and the baseline following the equation:
These maps were then statistically analyzed in a group analysis using Fieldtrip’s ft_frequencystatistics function (method: “depSamplesT”) to compute paired contrasts between each participant’s active stimulation condition versus sham. The resulting maps were corrected for multiple comparisons by the FDR method.
Statistical analyses
Inferential statistics were conducted using SAS JMP Pro (Version 14.1, Cary NC, USA). In order to assess stimulation-induced changes with our experimental factors of interest, we performed a baseline normalization step by computing, for each session, the difference between the post-stimulation power with the respective value at baseline (note, that in a preliminary step, in order to assess whether values at baseline were not significantly different across individuals and sessions, baseline power was assessed for session-wise differences. No significant effects were observed (Table S2)). Baseline-normalized data were entered into a linear mixed model (LMM), which allowed us to flexibly model the fixed effects of the experimental factors PROTOCOL and TIME, as well as the respective interaction. The use of LMMs over ANOVAs was also motivated by their ability to model effects of repeated measures over time with all available data, and independent from assumptions of normality of the underlying data [30]. In all models, restricted maximum likelihood estimation (REML) was used and a compound symmetry or unstructured covariance matrix. “Subjects” was denoted as a random factor, with random intercepts. The influence of fixed effects on the model was assessed using F tests. The alpha level for all tests was set to 0.05, and significant main effects or interactions were further analyzed by post-hoc two-tailed t-tests, corrected for multiple comparisons using the false discovery rate (FDR). In addition, exploratory analyses were also conducted using separate LMMs to assess the interaction of subject-level covariates, such as gender, age, intensity of stimulation (active motor threshold, AMT), baseline 10 Hz power, and the relative time of day of the experiment (i.e., morning, noon, afternoon). Findings from these analyses are described in Table S3.
Finite Element Method (FEM) simulation of TMS and tACS electric fieldsThe electrical field distribution resulting from both types of stimulation was calculated with the free Finite Element modeling software SimNIBS v3.0 [31] on a template head model provided in the example dataset (ernie.msh). Tissues were assigned homogeneous isotropic conductivities in the DC range (white matter: 0.15 S/m, gray matter: 0.4 S/m, CSF: 1.79 S/m, eyeballs and scalp: 0.33 S/m, skull: 0.008 S/m).
The TMS total electric field was obtained by adding the electric field components resulting from the effect of each coil independently. These were modeled with a 70 mm Figure-of-eight shaped coil, placed over F3 and F4 at 4 mm distance from the scalp (in order to account for the thickness of the tACS electrodes), and under a current rate of change of 1A/μs.
For tACS, four 2 cm-radius round electrodes with a 1 mm thickness for the electrode layer and 4 mm thickness for the gel (conductivity 8 S/m) were positioned on F3, F4, TP9 and TP10. The effect was calculated under the quasistatic approximation [32] only at the peak value of the maximally excitatory current on the DLPFC, i.e., with +0.5 mA at F3/F4 and -0.5 mA at TP9/TP10. The normal component of the electrical field on the gray matter can be derived from the current density vector by dividing by the conductivity of the gray matter.
Results and discussion
Statistical analysis was conducted based on our a priori hypothesis that prefrontal 10 Hz activity should be enhanced following combined stimulation, and when compared against each protocol on its own, and sham. Linear mixed model analyses indicated no significant differences at baseline between protocols in absolute 10 Hz prefrontal power in either EO or EC conditions at the group level (all values of p > 0.05; Table S2), and also good intra-subject reliability (intra-class correlation ICC(3,k): EO = 0.971 and EC = 0.862; Table S3). With respect to stimulation-induced changes (summarized in Fig. 2B–C), significant main effects were observed for the fixed factors PROTOCOL (EO: F(5,507.4) = 11.667, p < 0.0001; EC: F(5,507.8) = 11.429, p < 0.0001) and TIME (EO: F(3,503.6) = 13.819, p < 0.0001; EC: F(3,503.1) = 8.338, p < 0.0001). Post-hoc comparisons showed that in both resting-state conditions, peak-synchronized rTMS + tACS enhanced 10 Hz prefrontal power to the largest degree relative to baseline (44.64% during EO, 46.30% during EC; Fig. 2C), and also relative to uncombined, single protocols (rTMS alone and tACS alone), and relative to sham (two-tailed paired t-tests, p < 0.05, Tukey-corrected; see Supplementary Table S4 for all contrasts). These effects persisted for up to 30 min after the end of stimulation (Fig. 2B). Equivalent effects were observed when the same protocol was applied with closed eyes (42.59% increase during EO, 43.12% increase during EC). Interestingly, synchronized rTMS + tACS at the negative peak (trough) did not alter 10 Hz power when compared with baseline or sham, indicating a criticality in the timing of pulses. During EO but not EC resting-state, rTMS alone induced a 25.24% increase in 10 Hz power, which was significant only at the 1-min post-stimulation timepoint when compared with sham, and during EC, tACS alone induced an overall 17.73% increase in 10 Hz power, which was only significant relative to sham at the 1-min post-stimulation timepoint.
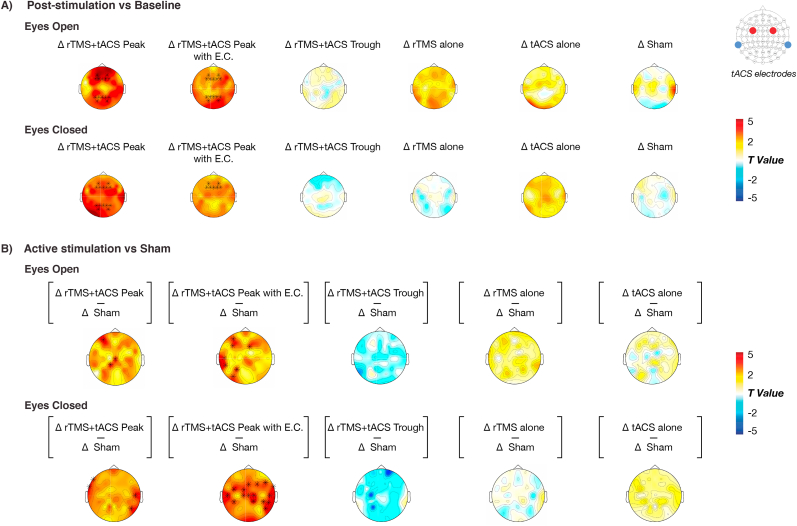
In additional confirmatory analyses, stimulation-induced changes in EEG were also analyzed across the frequency and spatial dimension to assess whether 1) changes were specific to the 10 Hz band; and 2) changes were specific to the prefrontal areas. With regard to frequency specificity, an interaction between Protocol x Frequency was observed during both EO (F(220,5495.7) = 1.231, p = 0.0125) and EC (F(220,5490.9) = 1.399, p < 0.0001). During EO, post-hoc comparisons revealed an increase in both 10- and 11 Hz power for peak-synchronized rTMS + tACS, and increased 10 Hz power for peak-rTMS + tACS + EC relative to sham (two-tailed t-tests, p < 0.05, corrected; Fig. 2D). In the EC condition, post-hoc comparisons revealed increased 10 Hz power for peak-rTMS + tACS + EC relative to sham and increased power at 10, 11, and 12 Hz power, as well as reduced 2, 3, and 4 Hz power for peak-synchronized rTMS + tACS (all values of p < 0.05, corrected; Fig. 2D). Spectral changes were also reanalyzed using irregular-resampling auto-spectral analysis (IRASA) [33], which confirmed that changes in 10 Hz activity reflected true changes in neural oscillations, and not merely aperiodic (1/f) activity (Figure S2). A whole-brain analysis also indicated modulation of network activity, as both peak-synchronized rTMS + tACS protocols induced increases in 10 Hz power over frontal and parietal regions in both EO and EC conditions, when contrasted against pre-stimulation baseline (Fig. 3A) and sham (Fig. 3B; p < 0.05, corrected). Finally, an additional linear mixed model was conducted to explore whether subject- or session-specific variables had an impact on stimulation-induced changes in power. The results showed that for both EO and EC conditions, subject age, gender, AMT, baseline 10-Hz power and the session’s time of day did not covary with stimulation effects (all values of p > 0.05; Table S3).
Fig. 3.
Whole-brain effects of stimulation. A) Whole brain, sensor level t-contrasts comparing each protocol’s grand-average post-stimulation 10 Hz activity with baseline activity. Asterisks indicate those EEG sensors surviving multiple comparison correction (p < 0.05, corrected). B) Whole brain, sensor level t-contrasts comparing each active stimulation protocol’s grand average change in 10 Hz activity (from baseline) with sham stimulation. Asterisks indicate those EEG sensors surviving multiple comparison correction (p < 0.05, corrected).
Taken together, our findings provide a demonstration of phase-synchronizing TMS with weak AC stimulation in order to induce and stabilize frequency-specific cortical oscillations in humans. The precise mechanisms underlying these after-effects require further exploration. With regard to tACS, data from animal and human experiments have suggested neural entrainment as a candidate mechanism for online effects [20,21,34,35]. A second, but not necessarily mutually exclusive contribution of the observed after-effects may be a tACS-induced facilitation of long term potentiation (LTP)-like plasticity of 10-Hz rTMS. In-silico modeling and simulation have suggested these effects may arise through spike-timing dependent plasticity (STDP) in the local network, as has been observed by tACS studies on the visual and motor cortices [4,5,23,24,36,37] as well as in combined rTMS and tACS studies, as mentioned in the introduction [18,19]. In support, studies using pharmacological manipulation of plasticity-related neurotransmitters have shown that both rTMS and tACS modulate neuroplastic changes in cortical excitability, which appear to be dependent on plasticity related mechanisms [6,23,36,38]. Finally, an interdependent contribution of both neural entrainment and plasticity cannot be ruled out since neural synchronization has been observed to be critical for establishing local and global synaptic plasticity [39,40]. In line with this observation, a tACS-EEG study observed that offline, after-effects of tACS correlated with the strength of online entrainment [22]. An analysis of the online effects of stimulation was not possible here, due to the technical limitations that prevent a straightforward removal of stimulation artifacts from online recordings [28,29,41]. Alternatively, further studies using pharmacological modulation may be informative to assess whether the after-effects observed by the dual-synchronization are directly related to synaptic plasticity mechanisms, such as NMDA receptor activation [6].
A particularly informative finding was that stimulation at the positive, but not the negative peak of tACS was critical for observing oscillatory after-effects. A natural explanation may be that in the peak tACS condition, the direction (normal component) of the induced electric field of tACS is excitatory according to current models [32], so that both rTMS and tACS effects are aligned in time in the targeted region, thereby producing an “in-phase” electric field as compared to the trough condition (Fig. 1C). We speculate that such alignment may be optimal for enhancing the LTP-like neuroplastic response to the combined rTMS and tACS application. In line with this view, a combined tACS + cTBS study by Goldsworthy et al. [18] observed that theta burst pulses applied to the trough of the tACS waveform resulted in facilitating the subsequent LTD-like depression of cortical excitability.
We note some limitations to the current study. First, the smaller sample size in the trough condition (n = 13) compared to the main experimental conditions (n = 25) may affect the interpretation of an absence of effect for this protocol due to the difference in statistical power. Although retrospective calculations with the obtained results lead us to have confidence that the difference between rTMS at the peak is significantly different than rTMS at the trough of tACS (Table S5), this issue should be resolved in future replications with larger sample sizes. Second, we limited our stimulation protocol to 8 min due to an abundance of caution towards safety and technical considerations of our TMS hardware. Previous studies using tACS have stimulated for up to 10–20 min and found comparable change in after-effects of alpha oscillations when using a posterior parietal-occipital montage [5,16,23,42]. Since alpha oscillatory tone is much less in frontal cortical areas, it remains to be seen whether increasing the stimulation duration for this montage may lead to an even greater enhancement in power.
In conclusion, we demonstrate feasibility of combining and synchronizing rTMS and tACS--two of the most widely used protocols for non-invasive neuromodulation of neural oscillations [36], and observe that phase-synchronized stimulation induces after-effects in oscillatory activity at the targeted frequency range. These observations offer promising perspectives for further exploration, such as the duration of the after-effects, and their functional importance. Moreover, these findings, in principle, suggest a novel approach for research into exploring cognitive processes through direct modulation of oscillatory activity, as well as clinical translation to pathological oscillations. Particularly, enhancing alpha oscillations over the frontal cortex may be a viable therapeutic approach in major depression, where frontal alpha activity is asymmetric [43], or in psychotic disorders, where frontal alpha activity is reduced [44].
CRediT authorship contribution statement
Tiam Hosseinian: Investigation, Formal analysis, Validation, Writing – review & editing. Fatemeh Yavari: Investigation, Methodology, Writing – review & editing. Maria Chiara Biagi: Methodology, Software, Writing – review & editing. Min-Fang Kuo: Investigation, Supervision, Project administration, Writing – review & editing. Giulio Ruffini: Methodology, Software, Supervision, Writing – review & editing. Michael A. Nitsche: Conceptualization, Funding acquisition, Project administration, Validation, Writing – review & editing, Supervision. Asif Jamil: Conceptualization, Investigation, Methodology, Formal analysis, Software, Validation, Supervision, Visualization, Writing – original draft, Writing – review & editing, Project administration.
Declaration of competing interest
All other authors have no conflict of interest.
M.A.N is a member of the advisory board of Neurolectrics
G.R. is a co-founder of Neuroelectrics.
Acknowledgements/Funding
We appreciate the assistance of Tobias Blanke, Nina Abich, and Lutger Blanke for their technical expertise and input. This work was funded by European Union’s Horizon 2020 research and innovation programme 2014–2018 under grant agreement number 686764 (“LUMINOUS").
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.brs.2021.03.011.
Contributor Information
Michael A. Nitsche, Email: nitsche@ifado.de.
Asif Jamil, Email: ajamil1@mgh.harvard.edu.
Author contributions
AJ, MFK and MAN conceived and supervised the project. MCB and GR conducted computational modeling. FY and AJ designed the experimental methodology. TH and AJ conducted experimental work, including all EEG recordings. TH and AJ conducted data analyses. TH and AJ wrote the manuscript, and all authors discussed the results and commented on the manuscript at all stages.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Herrmann C.S., Strüber D., Helfrich R.F., Engel A.K. EEG oscillations: from correlation to causality. Int J Psychophysiol. 2016;103:12–21. doi: 10.1016/j.ijpsycho.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 2.Widge A.S., Miller E.K. Targeting cognition and networks through neural oscillations. JAMA Psychiatry. 2019;76:671. doi: 10.1001/jamapsychiatry.2019.0740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thut G., Veniero D., Romei V., Miniussi C., Schyns P., Gross J. Rhythmic TMS causes local entrainment of natural oscillatory signatures. Curr Biol. 2011;21:1176–1185. doi: 10.1016/j.cub.2011.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zaehle T., Rach S., Herrmann C.S. Transcranial alternating current stimulation enhances individual alpha activity in human EEG. PLoS One. 2010;5:1–7. doi: 10.1371/journal.pone.0013766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kasten F.H., Dowsett J., Herrmann C.S. Sustained aftereffect of α-tACS lasts up to 70 min after stimulation. Front Hum Neurosci. 2016;10:1–9. doi: 10.3389/fnhum.2016.00245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wischnewski M., Engelhardt M., Salehinejad M.A., Schutter D.J.L.G., Kuo M.-F., Nitsche M.A. NMDA receptor-mediated motor cortex plasticity after 20 Hz transcranial alternating current stimulation. Cerebr Cortex. 2018;1–8 doi: 10.1093/cercor/bhy160. [DOI] [PubMed] [Google Scholar]
- 7.Bai Y., Xia X., Li X. A review of resting-state electroencephalography analysis in disorders of consciousness. Front Neurol. 2017;8:471. doi: 10.3389/fneur.2017.00471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bragin A., Engel J., Staba R.J. High-frequency oscillations in epileptic brain. Curr Opin Neurol. 2010;23:151–156. doi: 10.1097/WCO.0b013e3283373ac8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ali M.M., Sellers K.K., Fröhlich F. Transcranial alternating current stimulation modulates large-scale cortical network activity by network resonance. J Neurosci. 2013;33:11262–11275. doi: 10.1523/JNEUROSCI.5867-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Antal A., Herrmann C.S. Transcranial alternating current and random noise stimulation: possible mechanisms. Neural Plast. 2016;2016 doi: 10.1155/2016/3616807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hamada M., Murase N., Hasan A., Balaratnam M., Rothwell J.C. The role of interneuron networks in driving human motor cortical plasticity. Cerebr Cortex. 2013;23:1593–1605. doi: 10.1093/cercor/bhs147. [DOI] [PubMed] [Google Scholar]
- 12.Sommer M., Wu T., Tergau F., Paulus W. Intra- and interindividual variability of motor responses to repetitive transcranial magnetic stimulation. Clin Neurophysiol. 2002;113:265–269. doi: 10.1016/S1388-2457(01)00726-X. [DOI] [PubMed] [Google Scholar]
- 13.Maeda F., Keenan J.P., Tormos J.M., Topka H., Pascual-Leone A. Interindividual variability of the modulatory effects of repetitive transcranial magnetic stimulation on cortical excitability. Exp Brain Res. 2000;133:425–430. doi: 10.1007/s002210000432. [DOI] [PubMed] [Google Scholar]
- 14.Fekete T., Nikolaev A.R., De Knijf F., Zharikova A., van Leeuwen C. Multi-electrode alpha tACS during varying background tasks fails to modulate subsequent alpha power. Front Neurosci. 2018;12:428. doi: 10.3389/fnins.2018.00428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Veniero D., Benwell C.S.Y., Ahrens M.M., Thut G. Inconsistent effects of parietal α-tACS on Pseudoneglect across two experiments: a failed internal replication. Front Psychol. 2017;8:952. doi: 10.3389/fpsyg.2017.00952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kasten F.H., Duecker K., Maack M.C., Meiser A., Herrmann C.S. Integrating electric field modeling and neuroimaging to explain inter-individual variability of tACS effects. Nat Commun. 2019;10:1–11. doi: 10.1038/s41467-019-13417-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Doeltgen S.H., Mcallister S.M., Ridding M.C. Simultaneous application of slow-oscillation transcranial direct current stimulation and theta burst stimulation prolongs continuous theta burst stimulation-induced suppression of corticomotor excitability in humans. Eur J Neurosci. 2012;36:2661–2668. doi: 10.1111/j.1460-9568.2012.08181.x. [DOI] [PubMed] [Google Scholar]
- 18.Goldsworthy M.R., Vallence A.M., Yang R., Pitcher J.B., Ridding M.C. Combined transcranial alternating current stimulation and continuous theta burst stimulation: a novel approach for neuroplasticity induction. Eur J Neurosci. 2016;43:572–579. doi: 10.1111/ejn.13142. [DOI] [PubMed] [Google Scholar]
- 19.Guerra A., Suppa A., Bologna M., D’Onofrio V., Bianchini E., Brown P. Boosting the LTP-like plasticity effect of intermittent theta-burst stimulation using gamma transcranial alternating current stimulation. Brain Stimul. 2018;11:734–742. doi: 10.1016/j.brs.2018.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fröhlich F., McCormick D.A. Endogenous electric fields may guide neocortical network activity. Neuron. 2010;67:129–143. doi: 10.1016/j.neuron.2010.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Helfrich R.F., Schneider T.R., Rach S., Trautmann-Lengsfeld S.A., Engel A.K., Herrmann C.S. Entrainment of brain oscillations by transcranial alternating current stimulation. Curr Biol. 2014;24:333–339. doi: 10.1016/j.cub.2013.12.041. [DOI] [PubMed] [Google Scholar]
- 22.Helfrich R.F., Knepper H., Nolte G., Strüber D., Rach S., Herrmann C.S. Selective modulation of interhemispheric functional connectivity by HD-tACS shapes perception. PLoS Biol. 2014;12 doi: 10.1371/journal.pbio.1002031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vossen A., Gross J., Thut G. Alpha power increase after transcranial alternating current stimulation at alpha frequency (α-tACS) reflects plastic changes rather than entrainment. Brain Stimul. 2015;8:499–508. doi: 10.1016/j.brs.2014.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wischnewski M., Engelhardt M., Salehinejad M.A., Schutter D.J.L.G., Kuo M.-F., Nitsche M.A. NMDA receptor-mediated motor cortex plasticity after 20 Hz transcranial alternating current stimulation. Cerebr Cortex. 2019;29:2924–2931. doi: 10.1093/cercor/bhy160. [DOI] [PubMed] [Google Scholar]
- 25.Miller E.K. The prefontral cortex and cognitive control. Nat Rev Neurosci. 2000;1:59–65. doi: 10.1038/35036228. [DOI] [PubMed] [Google Scholar]
- 26.Rossi S., Hallett M., Rossini P.M., Pascual-Leone A., Avanzini G., Bestmann S. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin Neurophysiol. 2009;120:2008. doi: 10.1016/j.clinph.2009.08.016. 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Voss U., Holzmann R., Hobson A., Paulus W., Koppehele-Gossel J., Klimke A. Induction of self awareness in dreams through frontal low current stimulation of gamma activity. Nat Neurosci. 2014;17:810–812. doi: 10.1038/nn.3719. [DOI] [PubMed] [Google Scholar]
- 28.Noury N., Hipp J.F., Siegel M. Physiological processes non-linearly affect electrophysiological recordings during transcranial electric stimulation. Neuroimage. 2016;140:99–109. doi: 10.1016/j.neuroimage.2016.03.065. [DOI] [PubMed] [Google Scholar]
- 29.Noury N., Siegel M. Analyzing EEG and MEG signals recorded during tES, a reply. Neuroimage. 2018;167:53–61. doi: 10.1016/j.neuroimage.2017.11.023. [DOI] [PubMed] [Google Scholar]
- 30.Gelman A., Hill J. 2006. Data analysis using regression and multilevel/hierarchical models. Cambridge university press. [DOI] [Google Scholar]
- 31.Thielscher A., Antunes A., Saturnino G.B. 2015. Field modeling for transcranial magnetic stimulation: a useful tool to understand the physiological effects of TMS? pp. 222–225. (Proc. Annu. Int. Conf. IEEE eng. Med. Biol. Soc. EMBS). 2015- November, Institute of Electrical and Electronics Engineers Inc. [DOI] [PubMed] [Google Scholar]
- 32.Ruffini G., Wendling F., Merlet I., Molaee-Ardekani B., Mekonnen A., Salvador R. Transcranial current brain stimulation (tCS): models and technologies. IEEE Trans Neural Syst Rehabil Eng. 2013;21:333–345. doi: 10.1109/TNSRE.2012.2200046. [DOI] [PubMed] [Google Scholar]
- 33.Wen H., Liu Z. Separating fractal and oscillatory components in the power spectrum of neurophysiological signal. Brain Topogr. 2016;29:13–26. doi: 10.1007/s10548-015-0448-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Neuling T., Ruhnau P., Fuscà M., Demarchi G., Herrmann C.S., Weisz N. Friends, not foes: magnetoencephalography as a tool to uncover brain dynamics during transcranial alternating current stimulation. Neuroimage. 2015;118:406–413. doi: 10.1016/j.neuroimage.2015.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Johnson L., Alekseichuk I., Krieg J., Doyle A., Yu Y., Vitek J. Dose-dependent effects of transcranial alternating current stimulation on spike timing in awake nonhuman primates. Sci Adv. 2020;6:1–9. doi: 10.1126/sciadv.aaz2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Veniero D., Vossen A., Gross J., Thut G. Lasting EEG/MEG aftereffects of rhythmic transcranial brain stimulation: level of control over oscillatory network activity. Front Cell Neurosci. 2015;9:477. doi: 10.3389/fncel.2015.00477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Heise K., Kortzorg N., Bicalho G. Brain stimulation evaluation of a modified high-definition electrode montage for transcranial alternating current stimulation ( tACS ) of pre-central areas. Brain Stimul. 2016;9:700–704. doi: 10.1016/j.brs.2016.04.009. [DOI] [PubMed] [Google Scholar]
- 38.Rajan T.S., Ghilardi M.F.M., Wang H.Y., Mazzon E., Bramanti P., Restivo D. Mechanism of action for rTMS: a working hypothesis based on animal studies. Front Physiol. 2017;8 doi: 10.3389/fphys.2017.00457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Traub R.D., Bibbig A., LeBeau F.E.N., Buhl E.H., Whittington M.A. Cellular mechanisms of neuronal population oscillations in the hippocampus in vitro. Annu Rev Neurosci. 2004;27:247–278. doi: 10.1146/annurev.neuro.27.070203.144303. [DOI] [PubMed] [Google Scholar]
- 40.Uhlhaas P.J., Singer W. Abnormal neural oscillations and synchrony in schizophrenia. Nat Rev Neurosci. 2010;11:100–113. doi: 10.1038/nrn2774. [DOI] [PubMed] [Google Scholar]
- 41.Kasten F.H., Negahbani E., Fröhlich F., Herrmann C.S. Non-linear transfer characteristics of stimulation and recording hardware account for spurious low-frequency artifacts during amplitude modulated transcranial alternating current stimulation (AM-tACS) Neuroimage. 2018;179:134–143. doi: 10.1016/j.neuroimage.2018.05.068. [DOI] [PubMed] [Google Scholar]
- 42.Neuling T., Rach S., Herrmann C.S. Orchestrating neuronal networks: sustained after-effects of transcranial alternating current stimulation depend upon brain states. Front Hum Neurosci. 2013;7:1–12. doi: 10.3389/fnhum.2013.00161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Leuchter A.F., Cook I.A., Hunter A.M., Cai C., Horvath S. Resting-state quantitative electroencephalography reveals increased neurophysiologic connectivity in depression. PLoS One. 2012;7 doi: 10.1371/journal.pone.0032508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Howells F.M., Temmingh H.S., Hsieh J.H., Van Dijen A.V., Baldwin D.S., Stein D.J. Electroencephalographic delta/alpha frequency activity differentiates psychotic disorders: a study of schizophrenia, bipolar disorder and methamphetamine-induced psychotic disorder. Transl Psychiatry. 2018;8:1–11. doi: 10.1038/s41398-018-0105-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.