Abstract
Paracoccidioidomycosis (PCM) is an endemic mycosis in Latin America caused by the thermodimorphic fungi of the genus Paracoccidioides spp. Paracoccidioides lutzii (PL) is one of the 5 species that constitute the Paracoccidioides genus. PL expresses low amounts of glycoprotein (Gp) 43 (PLGp43) and PLGp43 displays few epitopes in common with the P. brasiliensis (PB) immunodominant antigen PBGp43, which is commonly used for serological diagnosis of PCM. This difference in structure between the glycoproteins markedly reduces the efficiency of serological diagnosis in patients infected with PL. We previously demonstrated that peptide 10 (P10) from the PBGp43 induces protective immune responses in in vitro and in vivo models of PB PCM. Since, P10 has proven to be a promising therapeutic to combat PB, we sought to identify peptides in PL that could similarly be applied for the treatment of PCM. PL yeast cell proteins were isolated from PL: dendritic cell co-cultures and subjected to immunoproteomics. This approach identified 18 PL peptides that demonstrated in silico predictions for immunogenicity. Eight of the most promising peptides were synthesized and applied to lymphocytes obtained from peptide-immunized or PL-infected mice as well as to in vitro cultures with peptides or dendritic cells pulsed the peptides. The peptides LBR5, LBR6 and LBR8 efficiently promoted CD4+ and CD8+ T cell proliferation and dendritic cells pulsed with LBR1, LBR3, LBR7 or LBR8 stimulated CD4+ T cell proliferation. We observed increases of IFN-γ in the supernatants from primed T cells for the conditions with peptides without or with dendritic cells, although IL-2 levels only increased in response to LBR8. These novel immunogenic peptides derived from PL will be employed to develop new peptide vaccine approaches and the proteins from which they are derived can be used to develop new diagnostic assays for PL and possibly other Paracoccidioides spp. These findings identify and characterize new peptides with a promising therapeutic profile for future against this important neglected systemic mycosis.
Keywords: paracoccidioidomycosis, vaccine, peptide, Paracoccidioides lutzii, PCM
Introduction
Paracoccidioidomycosis (PCM) is one of the major systemic fungal diseases in Latin America, with the highest incidence occurring in Brazil, Colombia and Venezuela (1–3). PCM is caused by fungi of the genus Paracoccidioides, which is constituted by the phylogenetic complex Paracoccidioides brasiliensis and the species P. lutzii (PL) (4). The P. brasiliensis complex was recently molecularly separated as P. brasiliensis (PB) and three new species: P. americana, P. restrepiensis and P. venezuelensis (5, 6). Remarkably, PCM due to each of these 5 Paracoccidioides species causes disease manifestations that are indistinguishable from one another.
In Brazil, PCM is not a mandatory reportable disease. Hence, the actual incidence of PCM is unknown, but it is estimated that the annual incidence is increasing from 0.71 to 3.7 cases per 100,000 inhabitants between 1980 and 1999 (7). There are regions with higher rates of PCM, such as in Rondônia were there are 9.4 cases per 100,000 inhabitants, with two cities reporting incidences of ~40 cases per 100,000 inhabitants (8). Although the specific distribution of Paracoccidioides species has not been entirely clarified (9–11), Rondônia, Mato Grosso, Pará are states in the north and central-west regions of Brazil where PL has been well documented (4, 12). The true incidence of each Paracoccidioides species is difficult to establish in part due to the limited ability to diagnose the infection. The standard diagnostic of PCM requires a direct identification of the fungus in fresh biological material (sputum, lesion scraping or lymph node aspirate examination) followed by isolation of the fungus which is hard and can take more than 15 days for the fungus to grow (13). The glycoprotein Gp43 produced by PB (PBGp43) is one of the main serological markers used in the diagnosis of PCM (14–17). However, PL expresses low amounts of Gp43 and PLGp43 displays few epitopes in common with the immunodominant PBGp43, which markedly reduces the efficiency of serological diagnosis in patients infected with PL (18, 19).
In addition to being applied for serological diagnosis, PBGp43 has been explored as a therapeutic (20). In particular, the peptide 10 (P10) from the PBGp43 (20) induces protective immune responses in in vitro and in vivo models. P10 induces proliferation of cells T CD4+ Th1 and the expression of high levels of INF-γ and IL-2 (20). Administration of P10 either before or after the establishment of PB PCM produces a therapeutically protective effect in both immunosuppressed and immunocompetent mice (21–24). P10 requires concomitant administration of adjuvant for efficacy although diverse adjuvants produce a therapeutic effect with the peptide (25), including delivery as a P10-nanoparticle (26). However, these protection experiments have been carried out using the PB (Pb18) isolate. PLGp43 is an active glucanase with partial antigenic identify with PBp43 (18), but the specific sequence of P10 has important amino acids changes in the equivalent region in PLGp43. Hence, P10 is unlikely to lead to protection against PL.
Since, P10 has proven to be a promising therapeutic to combat PB PCM, we sought to identify peptides in PL that could similarly be applied for treatment (27, 28). To accomplish this, we used an immunoproteomic approach, which is a powerful tool for identification and characterization of molecules with potential to serve as biomarkers for diagnoses (29–33). We validated select identified peptides for their ability to activate macrophages and dendritic cells as well as their ability to stimulate the proliferation of lymphocytes from mice immunized with the peptides. Of the 8 peptides we identified and synthesized, 4 promoted the proliferation of CD4+ T cells in co-culture with dendritic cells. Additionally, 2 and 3 peptides were able to promote the proliferation of CD4+ and T CD8+ T cells, respectively, when the peptides were added to culture medium. These findings support the continued pursuit of immunogenic peptides as therapeutics to combat PL PCM.
Material and Methods
Fungal Strains
PL (Pb8334) was grown in Fava-Netto solid medium at 37°C for 7 days. Thereafter, cells were isolated and washed three times in PBS, and large and agglomerated yeast cells were discarded resulting in the collection of uniform small yeast cells. Yeast cells were then heat inactivated at 60°C for 2 hours.
Mice
All procedures were performed according to the guidelines of National Council of Ethics with Animals (CONCEA) and the protocols were approved by the Ethical Committee for Animal Use from Institute of Biomedical Sciences at University of Sao Paulo (CEUA N° 2185220219, approved in 04/28/2019) and at Albert Einstein College of Medicine (Einstein) under the animal use protocol n° 00001281.
Culture Cells
Bone Marrow-derived Dendritic Cells (BMDC) were generated using 6-8-week-old male BALB/c mice according to an established protocol (22, 34). Briefly, mice were euthanized, and their femurs and tibias removed and flushed with RPMI (Vitrocell, Campinas, Brazil) medium to release bone marrow cells. The cells were counted in a hemocytometer and were plated at 107 cells/plate/10ml in 90mm × 15mm Petri with RPMI supplemented with IL-4 (15ng/ml) (Invitrogen), rGM-CSF (30ng/ml) (Invitrogen), 20μg/ml gentamicin (Gibco BRL Life Technologies), and 10% fetal bovine serum (Gibco). On the third and fifth day, 10 mls of fresh medium were added to the plate with growth factors, and the BMDC were collected at day 7 for phagocytosis assays.
The J774 (MØ) macrophage is a lineage culture cell derived from a reticulum cell sarcoma that has been extensively used to study yeast cell phagocytosis (35). J774 obtained from the ATCC were cultured in RPMI 1640, with 10% fetal calf serum (FCS; Vitrocell), 20 μg/ml gentamicin (Gibco BRL Life Technologies, Grand Island, NY, United States), and 1% nonessential amino acids in 90mm × 15mm Petri plate.
Phagocytosis Assay
BMDC and MØ were plated at 107 cells/plate (TPP, 75 cm2; 40 flasks of BMDC and 20 flasks of MØ). The BMDC and MØ used for experimentation displayed > 95% viability as determined by staining with Trypan blue. Cells were activated with IFN-γ (156 U/ml) for 12 hours prior to use in phagocytosis assays. Heat inactivated PL yeast (Pb8334) were added in the culture at a ratio of 5x1 (yeast x cells) and incubated for 8 hours at 5% CO2 and 37°C. After phagocytosis, the cells were removed from the flasks using a cell scraper, transferred to 50 ml falcon tubes and centrifuged for 10 min at 300 g at 4°C. Supernatants were discarded and pellets washed 2 times with PBS. Cells were lysed using buffer Tris [HCl (pH 7,6), 150mM NaCl com 1% de Igepal-CA630 (Sigma) supplemented with protease inhibitor (Roche)] for 2 hours at 4°C. After lysis, the samples were centrifuged for 10 min at 1,300g and 4°C. The supernatants were collected and centrifuged for 1 hour at 100,000g. The supernatants were collected and subjected to an immunoprecipitation assay.
Immunoprecipitation Assay
The protocol of immunoprecipitation was performed using Protein G (Dynabeads Protein G Immunoprecipitation Kit; Thermo Fisher Scientific). Briefly, the anti-MHC-II antibody (Clone KH74 and AF6-120.1; BioLegend) was linked to magnetic beads in the proportion 50 µg antibody to 200 µl of beads. To avoid the elution of the complex MHC-II + peptide, the beads were crosslinked with 5 mM BS3 diluted in Buffer Conjugation (20mM Na3PO4, 0.15M NaCl, pH 7.9). The Crosslink was stopped using Quenting Buffer (1 M Tris HCl, pH 7.5), and the beads were washed 3 times with PBS with 0,02% of Tween20. After placing the sample in a magnetic bookcase (DynaMag™-2, Invitrogen by Thermo Fisher Scientific) the supernatant was discarded and anti-MHC-II was added to the sample beads. The tube was removed from the magnetic and the sample was incubated for 30 min with agitation at room temperature. After incubation, the tube was again placed in the magnetic bookcase. The supernatant was discarded, and the pellet washed 3 times with Washing Buffer of the kit. After the last wash, the tube was removed from the magnetic, the supernatant discarded and Elution Buffer (50mM glycine, pH 2,8) was added to elute of the MHC-II-Peptide complex. The sample was incubated for 2 min with agitation at room temperature, placed into the magnetic bookcase and then the supernatant was collected for analysis by mass spectrometry.
Mass Spectrometry and Data Analysis
The samples generated by the immunoprecipitation were analyzed in the Proteomic Unit of the Department of Biochemistry of the Institute of Chemistry of the Federal University of Rio de Janeiro (UFRJ). Samples were trypsinized (Promega), desalted by C-18 spin column (Havard Apparatus) and dried in a vacuum centrifuge. The peptides resulted were resuspended in 0.1% formic acid and analyzed using an EASY-nLC system (Thermo Scientific) coupled to LTQ - Orbitrap Velos mass spectrometer (Thermo Scientific). The samples loaded onto a precolumn (ReprosilPur C18, 2 cm × 150 μm i. d. × 5 μm) with a flow rate of 5 μl/min and separated on the analytical column (ReprosilPur C18, 30 cm × 75 μm i.d. × 1.7 μm) with a gradient from 100% mobile phase A (0.1% FA) to 34% phase B (0.1% FA, 95% ACN) during 60 min, 34%–95% in 15 min and 5 min at 95% phase B at a constant flow rate of 250 nL/min. The LTQ-Orbitrap Velos was operated in positive ion mode with data-dependent acquisition. The full scan was obtained in the Orbitrap with an automatic gain control (AGC) target value of 10e6 ions and a maximum fill time of 500 ms. Each precursor ion scan was acquired at a resolution of 60,000 FWHM in the 400–1500 m/z mass range. Peptide ions were fragmented by CID MS/MS using a normalized collision energy of 35, target value of 2e5 ions and maximum fill time of 100ms. The 20 most abundant peptides were selected for MS/MS and dynamically excluded for a duration of 30s.
The raw data was processed with Proteome discoverer 2.1 software (Thermo Scientific). Peptides identifications were performed using the Sequest HT algorithm against FASTA format databases, contained protein sequences of PL and Mus musculus (for exclusion) provide by Uniprot (https://www.uniprot.org/). The searches were performed with peptide mass tolerance of 10 ppm, MS/MS tolerance of 0.5 Da, tryptic cleavage specificity, 2 maximum missed cleavage sites, fixed modification of carbamidomethyl (Cys), oxidation (Met), acetyl (N-terminus) and phospho (Ser, Thr, Tyr). False discovery rates (FDR) were obtained using Target Decoy PSM selecting identifications with a q-value equal or to less than 0.01.
In Silico Prediction
For in silico prediction, we used MHC-II Binding Predictions (http://tools.iedb.org/mhcii/) software from the Immune Epitope Database and Analysis Resource (IEDB) (http://www.iedb.org/). This software enables predictions of interactions of our peptide structures with MHC-II on host cells. The predictions for each protein allow for the ranking of the binding capacity of each peptide characterized by mass spectrometry. This rank, as a percentage, is a mean between the prediction methods available in the database. A peptide with a rank less than 10% is considered likely to be presented by MHC-II (36). The three-dimensional (3-D) representation of the proteins and the location of the peptides in the proteins was achieved using I-Tasser (http://zhanglab.ccmb.med.umich.edu/I-TASSER/) and the figures were generated using PyMol version 2.3 software (http://pymol.org/2/).
Peptides Synthesis
The peptides predicted to efficiently bind MHC-II were chemically synthesized by Biomatik Corporation (Ontario, CA) with purity of ≥95% as determined by HPLC and mass spectrometry.
Presentation of Peptides to BMDC and MØ
To study the ability of BMDC and MØ to present the peptides, BMDC or MØ in the concentration of 106 cells/mL were seeded into 96-well plates in RPMI medium with 5, 10 or 20 µg/mL of each peptide or without peptide for 6h in CO2 at 37°C. After the incubation, cells were collected, stained with florescent antibody and analyzed by flow cytometry.
BMDC and MØ Flow Cytometer Analyze
BMDC and MØ incubated with or without the peptides (5, 10 or 20 µg/mL) were washed with FACS buffer twice. Then, supernatants were removed and FACS buffer with Live/Dead – BV450 (BD Horizon); MHC-II – PE-Cy7 (clone: M5/114.15.2; Biolegend); F4/80 – FITC (clone: BM8; Biolegend); CD11c – BV711 (clone: N418; Biolegend); CD80 – PE (clone: 16-10A1; eBioscience); CD86 – APC (clone: GK1.5; Biolegend) were added for immunophenotyping and assessment of activation. The cells were incubated for 30 min at 4°C in the dark, washed 2X with FACS buffer and fixed with 1% paraformaldehyde. Sample acquisition was performed on a flow cytometer FACS BD LSRII housed in the Flow Cytometry Core Facility at the Einstein.
Lymphoproliferation Assay
We performed two different experiments to assess for lymphoproliferation. In the first approach, BALB/c mice were immunized via the subcutaneous route with two different mixtures of 4 peptides (Mix1: LBR1, LBR2, LBR3, LBR4; Mix2: LBR5, LBR6, LBR7, LBR8) at the final concentration of 10 µg/ml of each peptide in Freud’s complete adjuvant. After 7 days of immunization the mice were euthanized, the spleens and lymph nodes were harvested. Splenocytes and lymphocytes were incubated 90x16 culture plate in RPMI 1640 for 2h at 37°C to enrichment of the lymphocytes. After incubation, non-adherent cells were collected and stained with 5 μM CFSE in sterile PBS with 2% of FBS at room temperature in the dark for 15 min. The labeling was stopped with cold FBS and the samples were incubated for 5min at 4°C in the dark. Then, the cells were washed 2X with RPMI 1640 and suspended in complete RPMI medium (1% non-essential amino acids, 1% sodium pyruvate, 10% Fetal Bovine Serum, 2mM L-glutamine, 50μM b-mercaptoethanol, 50μg/ml ciprofloxacin). The lymphocytes were counted in Neubauer’s chamber and 2x105 cells were plated in 96-well plate. After plating, the lymphocytes were stimulated or not with 10µg/ml of each peptide separately or the lymphocytes were co-cultured with DCs (5 lymphocytes: 1 DC) with or without each peptide. The cultures were incubated in CO2 at 37°C for 72h, and then the supernatants collected and frozen at -20°C for subsequent cytokine analyses. The lymphocytes and the lymphocytes in co-culture with DCs were stained with Live/Dead – BV450; MHC-II – PE-Cy7; B220 – PE-Cy7; CD3 – PE; CD4 – APC; CD8 – PerCP and CSFE – FITC and T cell proliferation was evaluated by flow cytometry using a FACS BD LSRII.
In the second approach, BALB/c mice were infected intratracheally (i.t.) with log phase PL (Pb8334) yeast that were maintained on Sabouraud agar for 7 days at 37°C. To prepare the inoculum used in the experiments, PL was harvested from the tubes and washed three times with phosphate buffered saline (PBS, pH 7.2). Thereafter, large and agglutinated yeast cells were separated by decanting, and the small isolated yeast cells were collected and counted by hemocytometer, yeast cells with a viability of >95% as determined by Trypan blue staining. For the procedure, animals were anesthetized intraperitoneally using ∼200μl of a solution containing 80 mg/kg ketamine and 10 mg/kg xylazine (both from União Química Farmacêutica, Brazil). Five minutes after the injection of the anesthetics, a small longitudinal skin incision was made in the neck to expose the trachea and 1x107 yeast cells in 50 μl of PBS was injected. The incision was sutured with 5-0 silk. After 15 days of infection the mice were euthanized, the spleens and lymph nodes were harvested. Splenocytes and lymphocytes were incubated 90x16 culture plate in RPMI 1640 for 2h at 37°C to enrichment of the lymphocytes. Then, the cells were washed 2X with RPMI 1640 and suspended in complete RPMI. The lymphocytes were counted in Neubauer’s chamber and 2x105 cells were plated in 96-well plate. After plating, the lymphocytes were stimulated or not with 10µg/ml of each peptide separately or the lymphocytes were co-cultured with DCs (5 lymphocytes: 1 DC) with or without each peptide. The cultures were incubated in CO2 at 37°C for 72h, and then the supernatants collected and frozen at -20°C for subsequent cytokine analyses.
Cytokine Detection by Cytometric Bead Array (CBA) and ELISA
For cytokine determinations, the supernatants from the lymphoproliferation assays were thawed and analyses were performed using the BD CBA Mouse Th1/Th2/Th17 Cytokine Kit (BD Bioscience, San Jose, CA, USA), which measures IL-2, IL-4, IL-6, IFN-γ, TNF-α and IL-17A. The samples were measured on a FACS BD LSRII and analyzed by FCAP Array 3 Software (BD Bioscience). The theoretical limits of detection for the kit Th1/Th2/Th17 are 0.1 pg/mL for IL-2, 0.03 pg/mL for IL-4, 1.4 pg/mL for IL-6, 0.5 pg/mL for IFN-γ, 0.9 pg/mL for TNF and 0.8 pg/mL for IL-17A.
For cytokine detection in the supernatant from the lymphoproliferation assay after the mice were infected with PL, we thawed the samples and assayed with the IL-2, and IFN-γ using ELISA kits (BD OpTeia, San Diego, CA, United States). The detection limits of the assays were as follow: 3.1 pg/ml for IL-2, 31.3 pg/ml for IFN-γ as determined by the manufacturer.
Statistical Analysis
All assays were performed at least twice on separate dates using triplicates. The murine immunization assays were performed on two independent dates with two groups (Mix1 and Mix2) using 5 mice per group each time. GraphPad Prism 8 software (San Diego, CA, United States) was used to run the statistical analysis. The results were expressed as mean values and standard deviations (SD) of the indicated values. The data were previously analyzed as regards normality by Shapiro-Wilk’s method and then One-way ANOVA with Tukey’s post-test and confirmed by “t” test were employed for parametric data. p values of ≤0.05 were used to indicate statistical significance.
Results
Mass Spectrometry Assay
Mass spectrometry analysis identified 18 PL peptides from the cultures of BMDCs and/or macrophages (MØ) with heat-inactivated PL (Pb8334) yeast ( Table 1 ). The identified peptides were associated with 15 proteins and their respective genes ( Table 1 ). After protein identification, we use the FASTA format of PL deposited in the Uniprot (https://www.uniprot.org/) and MaxQuant software (https://www.maxquant.org/) to perform in silico predictions to determine percent rank for peptide binding to MHC-II according to the Immune Epitope Database and Analysis Resource (IEDB) consensus method described above. The in-silico prediction identified only one peptide (ITVTSQSAFDAQFNR) with a rank lower than 10% ( Table 1 ).
Table 1.
Proteins and peptides characterized by mass spectrometry after being presented by BMDC and/or MØ, and their IEDB rank.
| aa Quantitate | Sequenc | Protein name – Access name | Presented by: | IEDB Rank (%) |
|---|---|---|---|---|
| 14 | VPPAIAQFQNTLDR | 60S ribosomal protein L8-B –PAAG_04998 | BMDC | 63.5% |
| 11 | NFGIGQDIQPK | 65.0% | ||
| 16 | LKVPPAIAQFQNTLDR | 54.0% | ||
| 11 | QVHPDTGISNR | Histone H2B – PAAG_08918 | BMDC | 40.5% |
| 15 | AMSILNSFVNDIFER | 63.5% | ||
| 12 | DAGTIAGLNVLR | Hsp70-like protein –PAAG_01262 | BMDC | 28.1% |
| 25 | QHPSELETAIAGALSDLEANTPDLK | 40S ribosomal protein S7 – PAAG_07182 | BMDC | 22.0% |
| 11 | VNIGQILLSVR | 60S ribosomal protein L10-B –PAAG_01052 | BMDC | 28.5% |
| 11 | KVLTIINANQR | Ribosomal protein L35 –PAAG_02889 | BMDC | 16.0% |
| 17 | AIGIQPTEEGTIAVTTK | Ribosomal_L28e domain-containing protein –PAAG_03664 | BMDC | 51.5% |
| 15 | LESGNFSWGSEGISR | 40S ribosomal protein S8 –PAAG_00264 | BMDC | 92.0% |
| 27 | NAALTVGNILPLGSVPEGTVVTNVEEK | 60S ribosomal protein L2 –PAAG_00430 | BMDC | 61.0% |
| 15 | ITVTSQSAFDAQFNR | H15 domain-containing protein – PAAG_12131 | BMDC | 9.7% |
| 13 | IPYFNAPIYLENK | H/ACA ribonucleoprotein complex subunit –PAAG_05102 | BMDC | 53.5% |
| 16 | EDATALLYADPHNPMR | SET domain-containing protein – PAAG_12069 | BMDC/MØ | 54.5% |
| 11 | RFVNVTLTGGK | 40S ribosomal protein S30 – PAAG_00206 | BMDC | 40.5% |
| 21 | ENGCIIFISGLEDGTLLVCSK | tRNA ligase – PAAG_02801 | BMDC/MØ | 90.0% |
| 17 | GPRDHPFYSMSTQADGK | Uncharacterized protein –PAAG_12107 | BMDC | 38.5% |
In addition to synthesizing and testing the peptide with the IEDB less than 10%, we also generated two peptides located in the protein Histone H2B, one peptide located in the Hsp70-like protein, two peptides 60S ribosomal protein for which three peptides were characterized, and two other peptides that showed IEDBs of 16 and 22%. The peptides selected were named LBR1, LBR2, LBR3, LBR4, LBR5, LBR6, LBR7 and LBR8 ( Table 2 ).
Table 2.
Peptides selected to be synthesized and how they were named.
| Protein name – Access name | IEDB Rank (%) | Peptide named/Sequence |
|---|---|---|
| 60S ribosomal protein L8-B –PAAG_04998 | 65.0% | LBR1/LKVPPAIAQFQNTLDR |
| 54.0% | LBR2/NFGIGQDIQPK | |
| Histone H2B – PAAG_08918 | 40.5% | LBR3/QVHPDTGISNR |
| 63.5% | LBR4/AMSILNSFVNDIFER | |
| Hsp70-like protein – PAAG_01262 | 28.1% | LBR5/DAGTIAGLNVLR |
| 40S ribosomal protein S7 – PAAG_07182 | 22.0% | LBR6/QHPSELETAIAGALSDLEANTPDLK |
| Ribosomal protein L35 – PAAG_02889 | 16.0% | LBR7/VNIGQILLSVR |
| H15 domain-containing protein – PAAG_12131 | 9.7% | LBR8/ITVTSQSAFDAQFNR |
3-D Prospecting of Protein
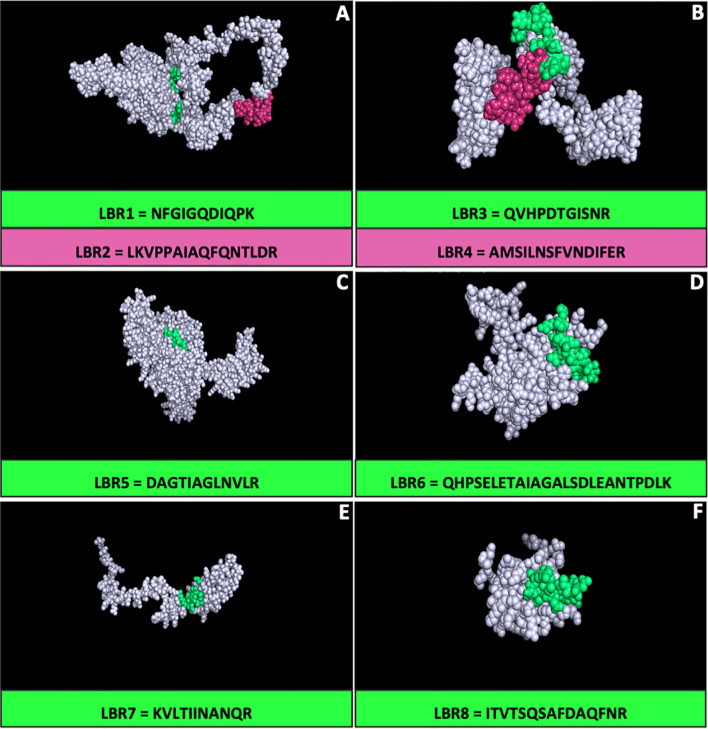
All proteins that had their respective peptides synthetized were analyzed three-dimensionally (3-D) to characterize the peptide positions in these proteins using the I-Tasser platform and the figures were visualized using the PyMol version 2.3 software ( Figure 1 ). The positions of each peptide were evaluated by 3-D modeling, particularly to determine whether they were presented on the surface. LBR2, LBR3, LBR4, LBR6 and LBR8 were the most exposed peptides.
Figure 1.
Three-dimensional (3-D) model of the proteins of Paracoccidioides lutzii by I-TASSER. The models were generated using peptides identified using immunoproteomics and the generated structure of their respective proteins. The figures and the peptide position in the protein were visualized using the PyMol software for (A) 60S ribosomal protein L8-B –PAAG_04998; (B) Histone H2B – PAAG_08918; (C) Hsp70-like protein – PAAG_01262; (D) 40S ribosomal protein S7 – PAAG_07182; (E) Ribosomal protein L35 – PAAG_02889; (F) H15 domain-containing protein – PAAG_12131.
Peptide Ability to Stimulate BMDC and MØ
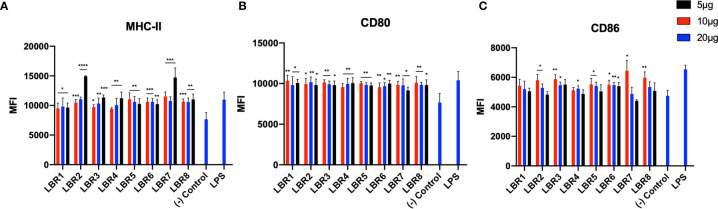
BDMC and MØ were pulsed with different concentrations of each peptide (5, 10 or 20µg/mL) for 6h ( Figures 2 and 3 ) and the cells were analyzed by flow cytometer to evaluate if the peptides increased the expression of costimulatory molecules on the cell membrane. Controls were untreated or LPS exposed. For BMDC, we observed that all peptides significantly increased the expression of MHC-II and CD80 molecules ( Figures 2A, B ). LBR1, LBR3, LBR5, LBR6 and LBR8 peptides also enhanced the expression of CD86 molecules ( Figure 2C ). The concentration of 10µg/mL of peptide generated the most consistent results ( Figure 2 ).
Figure 2.
The activation profiles of BDMC after being pulsed with peptides were analyzed by the mean fluorescence intensity (MFI) of the MHC-II (A), CD80 (B) and CD86 (C) molecules in the cells wall. These results are from three independent experiments. Data were analyzed by one-way ANOVA followed by Tukey’s post-test and confirmed by “t” test, where *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 in comparison to (-) Control group (culture of BMDC without stimulus).
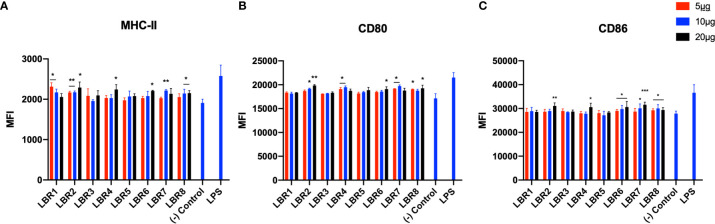
Figure 3.
The activation profiles of MØ after being pulsed with the peptides were analyzed by the mean fluorescence intensity (MFI) of the MHC-II (A), CD80 (B) and CD86 (C) molecules in the cells wall. These results are from three independent experiments. Data were analyzed by one-way ANOVA followed by Tukey’s post-test and confirmed by “t” test, where *p < 0.05, **p < 0.01, ***p < 0.001 and ****p < 0.0001 in comparison to (-) Control group (culture of MØ without stimulus).
Pulsing of MØ with LBR2, LBR4, LBR6, LBR7 or LBR8 significantly increased the expression of the CD80 and CD86 ( Figures 3B, C ). The expression of MHC-II ( Figure 3A ) significantly increased when MØ were pulsed with LBR1, LBR2, LBR4, LBR6, LBR7 or LBR8. Again, the concentration of 10µg/mL produced the most consistent results ( Figure 3 ).
In comparing the data between the BDMC and MØ, LBR2, LBR4, LBR6, LBR7 and LBR8 were the peptides that showed the best ability to stimulate both BMDC and/or MØ. Given the consistency of results with 10µg/mL peptide, we chose this concentration for murine immunization.
Lymphoproliferation Assay
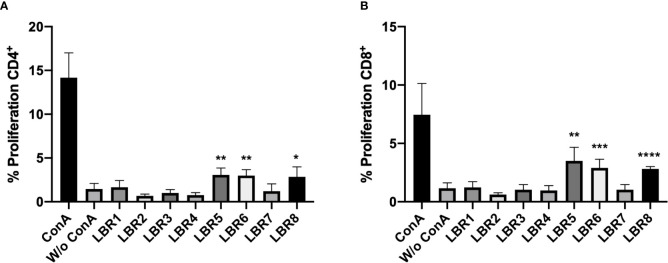
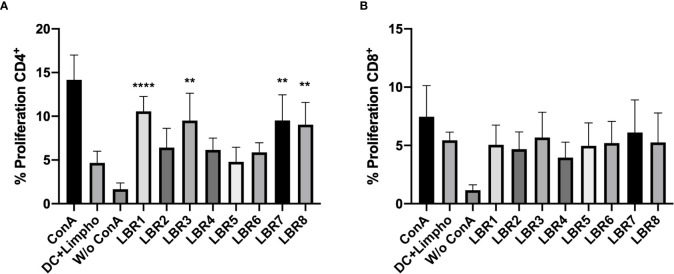
CD3+CD4+ and CD3+CD8+ T cells obtained from spleens and lymph nodes of mice after immunization with the peptides were subjected to proliferation assays. The LBR5 and BLR6 peptides significantly stimulated the proliferation of CD4+ T cells ( Figure 4A ), while CD8+ T Cells showed statistically increased proliferation when treated with LBR5, LBR6 or LBR8 peptides ( Figure 4B ).
Figure 4.
Proliferation of CD4+ (A) and CD8+ (B) T cells. Lymphocytes obtained from mice immunized with the mix of peptides (Mix1: LBR1, LBR2, LBR3, LBR4 or Mix2: LBR5, LBR6, LBR7, LBR8) were incubated with peptides for 72 h and T cell proliferation was analyzed by flow cytometry using the CFSE dilution method. Lymphocytes cultivated in the absence of a stimulus (W/o ConA) were used as a negative control and lymphocytes cultivated with Concanavalin A were used as positive control (ConA). The data shown are from two independent experiments. Data were analyzed by one-way ANOVA followed by Tukey’s post-test and confirmed by “t” test, where *p < 0.05, **p < 0.01, ***p < 0.001 and ****p < 0.0001 in comparison to unstimulated lymphocytes (W/o ConA).
We then assessed the ability of BMDC to present peptides to CD3+CD4+ and CD3+CD8+ T Cells ( Figure 5 ). In this system, BMDCs pulsed with the LBR1, LBR3, LBR7 or LBR8 significantly enhanced the proliferation of co-cultured CD4+ T cells when compared to unstimulated BMDCs ( Figure 5A ). CD8+ T cells proliferation was similar between co-cultures of unstimulated BDMCs and BMDCs pulsed with the peptides ( Figure 5B ).
Figure 5.
Proliferation of CD4+ (A) and CD8+ (B) T cells. BMDCs pulsed or not with a peptide were co-cultured with lymphocytes from mice immunized with the mix of peptides (Mix1: LBR1, LBR2, LBR3, LBR4 or Mix2: LBR5, LBR6, LBR7, LBR8) for 72 h and T cell proliferation was analyzed by flow cytometry using the CFSE dilution method. Lymphocytes cultivated in the absence of stimulus (W/o ConA) were used as negative control, lymphocytes cultivated with Concanavalin A were used as positive control (ConA) and co-cultures with BMDCs without peptide and lymphocytes without ConA (DC+Limpho) were the control groups. The data shown are from two independent experiments. Data were analyzed by one-way ANOVA followed by Tukey’s post-test and confirmed by “t” test, where **p < 0.01 and ****p < 0.0001 in comparison to co-culture of BMDCs and lymphocytes without stimulus (DC+Lympho).
Cytokine Production
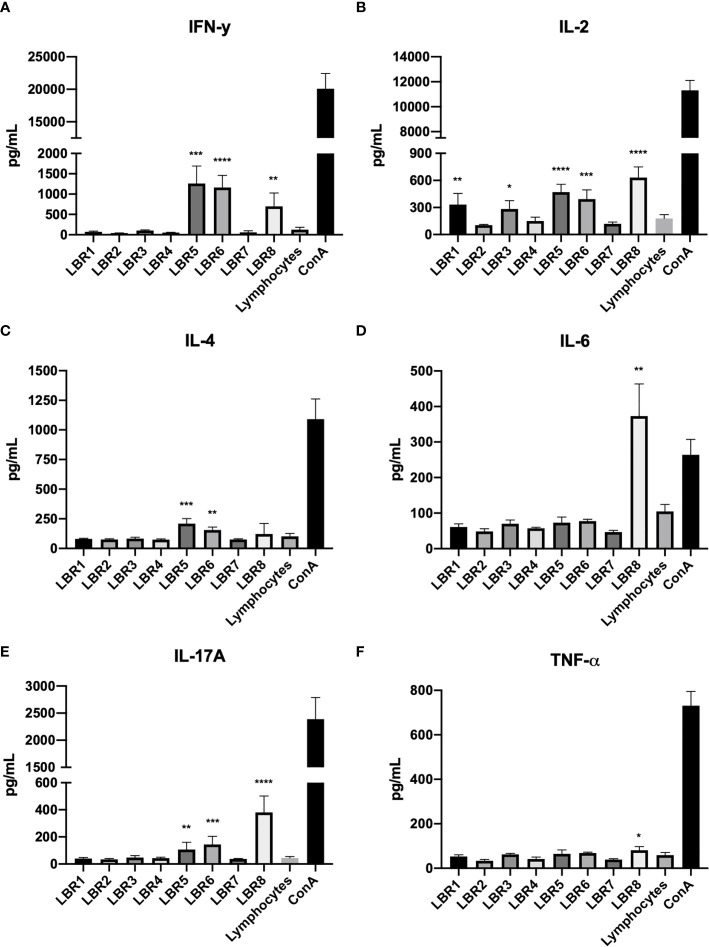
Cytokine concentrations of supernatants from the first approach of lymphoproliferation with the peptides and the co-cultures with BMDCs pulsed with the peptides were determined ( Figure 6 ). In the supernatants from the lymphoproliferation with the peptide LBR8, we observed significant increases of IFN-γ, IL-2, IL-6, IL-17A and TNF-α ( Figures 6A, B, D–F , respectively). LBR5 and LBR6 stimulated the significant increase of IFN-γ, IL-2, IL-4 and IL-17A ( Figures 6A–C, E , respectively) and we also observed the significant increase of IL-2 in the supernatants from assays with LBR1 and LBR3 ( Figure 6B ).
Figure 6.
Cytokine profiles in the supernatants of lymphoproliferation with the peptides (IFN-γ, IL-2, IL-4, IL6, IL17A and TNF-α cytokines represented in A–F respectively). The supernatants obtained from the lymphoproliferation after 72 h of incubation with the peptides were analyzed for cytokine levels by CBA. The supernatant from the lymphocytes cultivated in the absence of any kind of stimulus (Lymphocytes) was used as a negative control and the supernatant from the lymphocytes cultivated with Concanavalin A was used as a positive control (ConA). The data shown are from two independent experiments. Data were analyzed by one-way ANOVA followed by Tukey’s post-test and confirmed by “t” test, where *p < 0.05, **p < 0.01, ***p < 0.001 and ****p < 0.0001 in comparison to supernatant from the lymphocytes without stimulus (Lymphocytes).
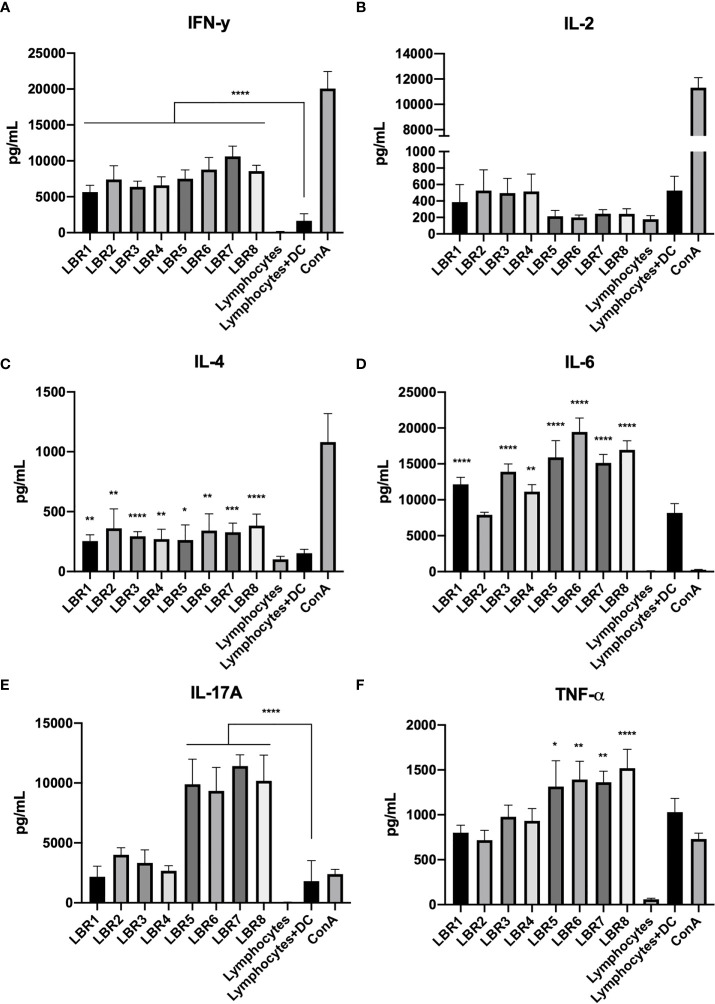
Cytokine measurements were also performed with supernatants obtained from the lymphocytes in co-culture with BMDCs pulsed with the peptides ( Figure 7 ). IL-4 and IFN-γ concentrations were increased in the supernatants from all conditions with peptide stimulation ( Figures 7A, C ). IL-6 was similarly increased, except for the condition with LBR2 ( Figure 7D ). Interestingly, IL-2 did not increase with exposure to any of the peptides ( Figure 7B ). TNF-α and IL-17A levels increased significantly in the LBR5, LBR6, LBR7 and LBR8 co-culture supernatants ( Figures 7E, F ).
Figure 7.
Cytokine profiles in the supernatant of co-culture lymphoproliferation (IFN-γ, IL-2, IL-4, IL6, IL17A and TNF-α cytokines represented in A–F respectively). The supernatants come from the co-cultures of lymphocytes (from peptide immunized mice) and BMDCs pulsed with peptides and proliferation was analyzed for cytokine levels by CBA. Lymphocytes cultivated in the absence of stimulus (Lymphocytes) were used as negative control, lymphocytes cultivated with Concanavalin A were used as positive control (ConA), and co-culture with BMDCs without be pulsed with peptide and lymphocytes without ConA (DC+Limpho) were the control groups. The data shown are from two independent experiments. Data were analyzed by one-way ANOVA followed by Tukey’s post-test and confirmed by “t” test, where **p < 0.01 and ***p < 0.001 in comparison to co-culture of BMDCs and lymphocytes without stimulus (DC+Lympho).
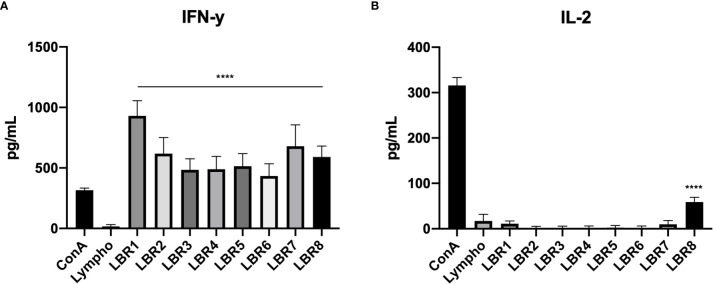
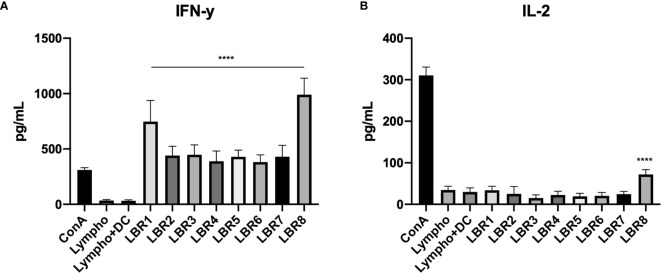
Measurements of cytokine levels were also performed with supernatant from lymphoproliferation of lymphocytes from PL-infected mice ( Figure 8 ), which showed significant increases of IFN-γ in the supernatant from all conditions with peptide stimulation when comparted with the control group which was the culture of lymphocytes only (Lympho) ( Figure 8A ). However, IL-2 levels increased significantly only when lymphocytes were exposed to LBR8 ( Figure 8B ).
Figure 8.
Cytokine profiles in the supernatants of lymphocytes from infected mice with the peptides (IFN-γ and IL-2 cytokines represented in A, B respectively). The supernatants obtained from the lymphoproliferation after 72 h of incubation with the peptides were analyzed for cytokine levels by ELISA. The supernatant from the lymphocytes cultivated in the absence of any kind of stimulus (Lymphocytes) was used as a negative control and the supernatant from the lymphocytes cultivated with Concanavalin A was used as a positive control (ConA). The data shown are from two independent experiments. Data were analyzed by one-way ANOVA followed by Tukey’s post-test and confirmed by “t” test, where ****p < 0.0001 in comparison to supernatant from the lymphocytes without stimulus (Lymphocytes).
Cytokine measurements were also performed with supernatant from lymphoproliferation of co-culture of lymphocytes from mice infected with DCs pulsed with peptides ( Figure 9 ). Results similar to those found in the supernatant of lymphoproliferation with peptides in the co-culture of lymphocytes from mice infected with DCs pulsed with peptides supernatant were observed. We observed significant increases of IFN-γ in the supernatant from all condition with DCs pulsed with peptides when compared with the control group, which were co-cultures of lymphocytes and DCs without peptide (Lympho + DC) ( Figure 9A ). IL-2 increased significantly only in response to LBR8 ( Figure 9B ).
Figure 9.
Cytokine profiles in the supernatant of co-culture lymphoproliferation (IFN-γ and IL-2 cytokines represented in A, B respectively). The supernatants come from the co-cultures of lymphocytes from infected mice and BMDCs pulsed with peptides and cytokine levels were analyzed by ELISE. Lymphocytes cultivated in the absence of stimulus (Lympho) were used as negative control, lymphocytes cultivated with Concanavalin A were used as positive control (ConA), and co-culture with BMDCs without be pulsed with peptide and lymphocytes without ConA (Limpho+DC) were the control groups. The data shown are from two independent experiments. Data were analyzed by one-way ANOVA followed by Tukey’s post-test and confirmed by “t” test, ****p < 0.0001 in comparison to co-culture of BMDCs and lymphocytes without stimulus (Lympho+DC).
Discussion
PCM is one the most important systemic mycosis in the Latin America, and the rates of infection with Paracoccidioides species have been increasing steadily over the past few years (7), especially within the new agricultural borders in Brazil, which are considered endemic to PL, in particular (9–12). The treatment of PCM presents several challenges that complicate effective patient care, including prolonged treatment time and a high incidence of relapses. Hence, there is an urgent need to identify new therapeutic approaches to combat this complex disease. One of the most promising treatment innovations for PCM is the inclusion of the peptide P10 in therapy, which is highlighted by P10’s ability to be used both early in or after disease in immunologically normal or compromised mice (21–24, 26). However, P10 does not sufficiently correspond to sequences in PLGp43. Hence, we sought to identify immunostimulatory peptides in PL that could be developed as therapeutics. During the performance of this project, we use bioinformatic and immunoproteomic tolls, they are potentially useful tool to identify disease-associated antigens that can be used to elicit the immune responses or in the diagnostic, both methodologies has been currently used in the paracoccidioimycosis context and they are presenting promisor results (28, 37).
In this study, we identified peptides from PL with a capacity to stimulate T cell responses using an immunoproteomic assay followed by in silico prediction. We found 18 peptides that met predictive criteria for having a capacity to be presented by MCH-II from BMDC and/or MØ. Three of these peptides came from the same protein [60S ribosomal protein L8-B, a protein synthase that is suppressed during phagocytosis (38)], which may suggest that this protein can be quite immunogenic. Another fact that may suggest that this protein can be immunogenic is that it is located in the fungal cell wall (39, 40). Two peptides were derived from Histone H2B, which is also found in the cell wall (39, 40). In Histoplasma capsulatum, cell wall H2B has been targeted with mAb’s, and opsonization by mAb to H2B alters the intracellular fate of the yeast and results in protection in a murine model of lethal histoplasmosis (41). One peptide is derived from Hsp70. Hsp70 is one of the highly recognized antigens by antibodies in sera of patients with either histoplasmosis or PCM, confirming the fungal expression and immunogenicity of HSP70 during infection (42–47). Peptides LBR1, LBR2, LBR3, LBR4, LBR5, LBR6, and LBR7 came from protein that have previously been identified in the fungal cell wall by Silva, R.P. and collaborators (40). LBR8 belongs to the Histone H1 protein, which is located in the cell nucleus, as describe in the UniProt.
The 8 peptides with the lowest IEDB ranks or derived from known cell wall expressed proteins were synthesized and tested in vitro to determine their ability to stimulate BDMC and MØ, and their capacity to stimulate lymphocytes proliferation was assessed. The PL peptides increased the expression of key superficial molecules (MCH-II, CD80 and CD86) on BDMC and MØ, which is consistent with a capacity of the peptides to change the activation of these cells. This is consistent with our findings with P10, which similarly increased the expression of these markers on BDMC (22–24). We also found that lymphocytes from mice immunized with a mix (Mix1 or Mix2) of peptides in Freund’s adjuvant proliferated significantly in response to each individual peptide. Similarly, DCs pulsed with each peptide efficiently stimulated lymphocyte proliferation. Specifically, peptides LBR5 and LBR6 induced CD4+ and CD8+ T lymphocyte proliferation and LBR8 induced CD8+ T lymphocyte proliferation. DCs pulsed LBR1, LBR2, LBR7 or LBR8 induced CD4+ T lymphocyte proliferation. Cytokine analyses revealed that these proliferative responses were characterized by a mix of Th1, Th2 and Th17 T cells, since we observed the production of IFN-γ, IL-2, IL-4, IL-6, TNF-α and IL-17A.
Immunoprotection in PCM is mediated by Th1/Th17 responses that are controlled by the anti-inflammatory activity of Treg cells (48). In contrast, severe forms of disease are associated with Th-2/Th-9 responses and excessive expansion of Treg cells. Our result demonstrated that LBR5, LBR6, and LBR8 promoted a mixed Th1, Th2, and Th17 response. However, LBR5, LBR6 and LBR8 induced increased levels of pro-inflammatory cytokines (IFN-γ, IL-2, IL-6, TNF-α and IL-17A), which are consistent with a robust Th1 immune response that especially promotes cellular proliferation and the formation of granulomas (IFN-γ and TNF-α) (22, 24, 49). Th17 immunity was also induced, as demonstrated by increased concentrations of IL-6 and IL-17A (50–52). Hence, our findings suggest that these peptides have the potential to be utilized in the treatment of PCM due to PL. They may also be useful in the diagnosis of the disease.
Remarkably, each of the PL peptides tested was able to induce proliferation of lymphocytes primed by previous immunization of mice with the peptides LBR5, LBR6 and LBR8. Calich and collaborators (53) in a susceptible-resistant experimental model correlated the specific antibody response and the polyclonal B-cell activation with progressive infection in the susceptible B10.A mice whereas resistant A/Sn mice developed a strong DTH response when challenged with PB with low levels of specific antibodies and no evidence of B cell activation (53). Taborda et al. (20) identified that the peptide (P10) as the immunodominant T cell epitope in PBGp43 as it was able to induce proliferation, including that of lympho-node CD4+ T cells of the Th1 subtype proliferation, forming INF-γ and IL-2 (20). In the present work, we found that lymphocytes from mice infected with PL (PB8334) and stimulated with LBR8 had cytokine profiles suggesting proliferation with significant increase of IL-2. We observed an immune response by CD4+ T cells subtype Th1 with production of IFN-γ in response to all 8 peptides. These results are similar to those found by Taborda and collaborators in 1998, when P10, the most promisor peptide to be used in the PCM treatment to date, was identified (20). These results suggest that the peptide LBR8 as well as the other immunostimulating peptides may also be applied therapeutically. Future studies will explore the effectiveness of the PL peptides as vaccines and examine whether the PL proteins associated with these peptides can be used as diagnostics.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://www.uniprot.org/, A0A0A2V091, https://www.uniprot.org/, C1GPI5, https://www.uniprot.org/, C1HDS5, https://www.uniprot.org/, C1H2V9, https://www.uniprot.org/, A0A0A2V0A9, https://www.uniprot.org/, C1GP19, https://www.uniprot.org/, C1GXU1, https://www.uniprot.org/, C1GRW7, https://www.uniprot.org/, C1H2K5, https://www.uniprot.org/, C1GNW1, https://www.uniprot.org/, C1H8U1, https://www.uniprot.org/, C1GRA7, https://www.uniprot.org/, C1GWA6, https://www.uniprot.org/, C1GWJ4.
Ethics Statement
All procedures were performed according to the guidelines of National Council of Ethics with Animals (CONCEA) and the protocols were approved by the Ethical Committee for Animal Use from Institute of Biomedical Sciences at University of Sao Paulo (CEUA ICB USP certificates 101/2014, approved in 01/12/2014) and at Albert Einstein College of Medicine (Einstein) under the animal use protocol n° 00001281.
Author Contributions
LS performed the experiments, analyzed the data, and wrote the manuscript. CT and LC performed proliferation experiments. MM and MJ performed the immunoproteomic assay. JN and CT conceived the project, designed the experiments, and wrote and revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This research was funded by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP), grants number 2016/08730-6, 2018/25171-6 and 2019/20622-2. CNPq (grant number 420480/2018-8), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – CAPES.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- 1. Brummer E, Castaneda E, Restrepo A. Paracoccidioidomycosis: An Update. Clin Microbiol Rev (1993) 6:89–117. 10.1128/cmr.6.2.89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Martinez R. Epidemiology of Paracoccidioidomycosis. Rev Inst Med Trop Sao Paulo (2015) 57 Suppl 19:11–20. 10.1590/S0036-46652015000700004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mendes RP, Cavalcante RS, Marques SA, Marques MEA, Venturini J, Sylvestre TF, et al. Paracoccidioidomycosis: Current Perspectives From Brazil. Open Microbiol J (2017) 11:224–82. 10.2174/1874285801711010224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Teixeira MM, Theodoro RC, de Carvalho MJ, Fernandes L, Paes HC, Hahn RC, et al. Phylogenetic Analysis Reveals a High Level of Speciation in the Paracoccidioides Genus. Mol Phylogenet Evol (2009) 52:273–83. 10.1016/j.ympev.2009.04.005 [DOI] [PubMed] [Google Scholar]
- 5. Bueno RA, Thomaz L, Munoz JE, da Silva CJ, Nosanchuk JD, Pinto MR, et al. Antibodies Against Glycolipids Enhance Antifungal Activity of Macrophages and Reduce Fungal Burden After Infection With Paracoccidioides Brasiliensis. Front Microbiol (2016) 7:74. 10.3389/fmicb.2016.00074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Turissini DA, Gomez OM, Teixeira MM, McEwen JG, Matute DR. Species Boundaries in the Human Pathogen Paracoccidioides. Fungal Genet Biol (2017) 106:9–25. 10.1016/j.fgb.2017.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Martinez R. New Trends in Paracoccidioidomycosis Epidemiology. J Fungi (Basel) (2017) 3(1):1. 10.3390/jof3010001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Vieira Gde D, Alves Tda C, Lima SM, Camargo LM, Sousa CM. Paracoccidioidomycosis in a Western Brazilian Amazon State: Clinical-Epidemiologic Profile and Spatial Distribution of the Disease. Rev Soc Bras Med Trop (2014) 47:63–8. 10.1590/0037-8682-0225-2013 [DOI] [PubMed] [Google Scholar]
- 9. Takayama A, Itano EN, Sano A, Ono MA, Kamei K. An Atypical Paracoccidioides Brasiliensis Clinical Isolate Based on Multiple Gene Analysis. Med Mycol (2010) 48:64–72. 10.3109/13693780902718065 [DOI] [PubMed] [Google Scholar]
- 10. Arantes TD, Theodoro RC, Da Graca Macoris SA, Bagagli E. Detection of Paracoccidioides Spp. in Environmental Aerosol Samples. Med Mycol (2013) 51:83–92. 10.3109/13693786.2012.698444 [DOI] [PubMed] [Google Scholar]
- 11. Arantes TD, Theodoro RC, Teixeira Mde M, Bosco Sde M, Bagagli E. Environmental Mapping of Paracoccidioides Spp. in Brazil Reveals New Clues Into Genetic Diversity, Biogeography and Wild Host Association. PloS Negl Trop Dis (2016) 10:e0004606. 10.1371/journal.pntd.0004606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Teixeira Mde M, Theodoro RC, Derengowski Lda S, Nicola AM, Bagagli E, Felipe MS. Molecular and Morphological Data Support the Existence of a Sexual Cycle in Species of the Genus Paracoccidioides. Eukaryot Cell (2013) 12:380–9. 10.1128/EC.05052-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Shikanai-Yasuda MA, Mendes RP, Colombo AL, Queiroz-Telles F, Kono ASG, Paniago AMM, et al. Brazilian Guidelines for the Clinical Management of Paracoccidioidomycosis. Rev Soc Bras Med Trop (2017) 50:715–40. 10.1590/0037-8682-0230-2017 [DOI] [PubMed] [Google Scholar]
- 14. Puccia R, Travassos LR. 43-Kilodalton Glycoprotein From Paracoccidioides Brasiliensis: Immunochemical Reactions With Sera From Patients With Paracoccidioidomycosis, Histoplasmosis, or Jorge Lobo’s Disease. J Clin Microbiol (1991) 29:1610–5. 10.1128/JCM.29.8.1610-1615.1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Camargo ZP, Gesztesi JL, Saraiva EC, Taborda CP, Vicentini AP, Lopes JD. Monoclonal Antibody Capture Enzyme Immunoassay for Detection of Paracoccidioides Brasiliensis Antibodies in Paracoccidioidomycosis. J Clin Microbiol (1994) 32:2377–81. 10.1128/JCM.32.10.2377-2381.1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Taborda CP, Camargo ZP. Diagnosis of Paracoccidioidomycosis by Dot Immunobinding Assay for Antibody Detection Using the Purified and Specific Antigen Gp43. J Clin Microbiol (1994) 32:554–6. 10.1128/JCM.32.2.554-556.1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zancope-Oliveira RM, Pizzini CV, de Medeiros Muniz M, do Valle ACF, Almeida-Paes R. Diagnostic Aspects of Paracoccidioidomycosis. Curr Trop Med Rep (2014) 1:111–8. 10.1007/s40475-014-0022-y [DOI] [Google Scholar]
- 18. Leitao NP, Jr., Vallejo MC, Conceicao PM, Camargo ZP, Hahn R, Puccia R. Paracoccidioides Lutzii Plp43 is an Active Glucanase With Partial Antigenic Identity With P. Brasiliensis Gp43. PloS Negl Trop Dis (2014) 8:e3111. 10.1371/journal.pntd.0003111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Batista J, Jr., de Camargo ZP, Fernandes GF, Vicentini AP, Fontes CJ, Hahn RC. Is the Geographical Origin of a Paracoccidioides Brasiliensis Isolate Important for Antigen Production for Regional Diagnosis of Paracoccidioidomycosis? Mycoses (2010) 53:176–80. 10.1111/j.1439-0507.2008.01687.x [DOI] [PubMed] [Google Scholar]
- 20. Taborda CP, Juliano MA, Puccia R, Franco M, Travassos LR. Mapping of the T-cell Epitope in the Major 43-Kilodalton Glycoprotein of Paracoccidioides Brasiliensis Which Induces a Th-1 Response Protective Against Fungal Infection in BALB/c Mice. Infect Immun (1998) 66:786–93. 10.1128/IAI.66.2.786-793.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Munoz JE, Luft VD, Amorim J, Magalhaes A, Thomaz L, Nosanchuk JD, et al. Immunization With P10 Peptide Increases Specific Immunity and Protects Immunosuppressed BALB/c Mice Infected With Virulent Yeasts of Paracoccidioides Brasiliensis. Mycopathologia (2014) 178:177–88. 10.1007/s11046-014-9801-1 [DOI] [PubMed] [Google Scholar]
- 22. Silva LBR, Dias LS, Rittner GMG, Munoz JE, Souza ACO, Nosanchuk JD, et al. Dendritic Cells Primed With Paracoccidioides Brasiliensis Peptide P10 Are Therapeutic in Immunosuppressed Mice With Paracoccidioidomycosis. Front Microbiol (2017) 8:1057. 10.3389/fmicb.2017.01057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Silva LBR, Taira CL, Dias LS, Souza ACO, Nosanchuk JD, Travassos LR, et al. Experimental Therapy of Paracoccidioidomycosis Using P10-Primed Monocyte-Derived Dendritic Cells Isolated From Infected Mice. Front Microbiol (2019) 10:1727. 10.3389/fmicb.2019.01727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Magalhaes A, Ferreira KS, Almeida SR, Nosanchuk JD, Travassos LR, Taborda CP. Prophylactic and Therapeutic Vaccination Using Dendritic Cells Primed With Peptide 10 Derived From the 43-Kilodalton Glycoprotein of Paracoccidioides Brasiliensis. Clin Vaccine Immunol (2012) 19:23–9. 10.1128/CVI.05414-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mayorga O, Munoz JE, Lincopan N, Teixeira AF, Ferreira LC, Travassos LR, et al. The Role of Adjuvants in Therapeutic Protection Against Paracoccidioidomycosis After Immunization With the P10 Peptide. Front Microbiol (2012) 3:154. 10.3389/fmicb.2012.00154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rodrigues Dos Santos Junior ,S, Kelley Lopes da Silva F, Santos Dias L, Oliveira Souza AC, Valdemir de Araujo M, Buffoni Roque da Silva L, et al. C, P.T. Intranasal Vaccine Using P10 Peptide Complexed Within Chitosan Polymeric Nanoparticles as Experimental Therapy for Paracoccidioidomycosis in Murine Model. J Fungi (Basel) (2020) 6(3):160. 10.3390/jof6030160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Moreira ALE, Oliveira MAP, Silva LOS, Inacio MM, Bailao AM, Parente-Rocha JA, et al. Immunoproteomic Approach of Extracellular Antigens From Paracoccidioides Species Reveals Exclusive B-Cell Epitopes. Front Microbiol (2019) 10:2968. 10.3389/fmicb.2019.02968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rodrigues AM, Kubitschek-Barreira PH, Pinheiro BG, Teixeira-Ferreira A, Hahn RC, de Camargo ZP. Immunoproteomic Analysis Reveals Novel Candidate Antigens for the Diagnosis of Paracoccidioidomycosis Due to Paracoccidioides Lutzii. J Fungi (Basel) (2020) 6(3):35. 10.3390/jof6040357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Virginio ED, Kubitschek-Barreira PH, Batista MV, Schirmer MR, Abdelhay E, Shikanai-Yasuda MA, et al. Immunoproteome of Aspergillus Fumigatus Using Sera of Patients With Invasive Aspergillosis. Int J Mol Sci (2014) 15:14505–30. 10.3390/ijms150814505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pitarch A, Nombela C, Gil C. Seroprofiling At the Candida Albicans Protein Species Level Unveils an Accurate Molecular Discriminator for Candidemia. J Proteomics (2016) 134:144–62. 10.1016/j.jprot.2015.10.022 [DOI] [PubMed] [Google Scholar]
- 31. Jobbins SE, Hill CJ, D’Souza-Basseal JM, Padula MP, Herbert BR, Krockenberger MB. Immunoproteomic Approach to Elucidating the Pathogenesis of Cryptococcosis Caused by Cryptococcus Gattii. J Proteome Res (2010) 9:3832–41. 10.1021/pr100028t [DOI] [PubMed] [Google Scholar]
- 32. Rodrigues AM, Kubitschek-Barreira PH, Fernandes GF, de Almeida SR, Lopes-Bezerra LM, de Camargo ZP. Immunoproteomic Analysis Reveals a Convergent Humoral Response Signature in the Sporothrix Schenckii Complex. J Proteomics (2015) 115:8–22. 10.1016/j.jprot.2014.11.013 [DOI] [PubMed] [Google Scholar]
- 33. Silva LBR, Taborda CP, Nosanchuk JD. Advances in Fungal Peptide Vaccines. J Fungi (Basel) (2020) 6(3):119. 10.3390/jof6030119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Inaba K, Inaba M, Romani N, Aya H, Deguchi M, Ikehara S, et al. Generation of Large Numbers of Dendritic Cells From Mouse Bone Marrow Cultures Supplemented With Granulocyte/Macrophage Colony-Stimulating Factor. J Exp Med (1992) 176:1693–702. 10.1084/jem.176.6.1693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pappagianis D, Levine HB. The Present Status of Vaccination Against Coccidioidomycosis in Man. Am J Epidemiol (1975) 102:30–41. 10.1093/oxfordjournals.aje.a112131 [DOI] [PubMed] [Google Scholar]
- 36. Wang P, Sidney J, Dow C, Mothe B, Sette A, Peters B. A Systematic Assessment of MHC Class II Peptide Binding Predictions and Evaluation of a Consensus Approach. PloS Comput Biol (2008) 4:e1000048. 10.1371/journal.pcbi.1000048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rosa SBA, Csordas BG, do Valle Leone de Oliveira SM, Santos ARD, Paniago AMM, Venturini J. Prediction of Conserved Peptides of Paracoccidioides for Interferon-gamma Release Assay: The First Step in the Development of a Lab-Based Approach for Immunological Assessment During Antifungal Therapy. J Fungi (Basel) (2020) 6(4):379. 10.3390/jof6040379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Parente-Rocha JA, Parente AF, Baeza LC, Bonfim SM, Hernandez O, McEwen JG, et al. Macrophage Interaction With Paracoccidioides Brasiliensis Yeast Cells Modulates Fungal Metabolism and Generates a Response to Oxidative Stress. PloS One (2015) 10:e0137619. 10.1371/journal.pone.0137619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Araujo DS, de Sousa Lima P, Baeza LC, Parente AFA, Melo Bailao A, Borges CL, et al. Employing Proteomic Analysis to Compare Paracoccidioides Lutzii Yeast and Mycelium Cell Wall Proteins. Biochim Biophys Acta Proteins Proteom (2017) 1865:1304–14. 10.1016/j.bbapap.2017.08.016 [DOI] [PubMed] [Google Scholar]
- 40. Peres da Silva R, Longo LGV, Cunha J, Sobreira TJP, Rodrigues ML, Faoro H, et al. Comparison of the RNA Content of Extracellular Vesicles Derived From Paracoccidioides Brasiliensis and Paracoccidioides Lutzii. Cells (2019) 8(7):765. 10.3390/cells8070765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Nosanchuk JD, Steenbergen JN, Shi L, Deepe GS, Jr., Casadevall A. Antibodies to a Cell Surface Histone-Like Protein Protect Against Histoplasma Capsulatum. J Clin Invest (2003) 112:1164–75. 10.1172/JCI19361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Rappleye CA, Goldman WE. Defining Virulence Genes in the Dimorphic Fungi. Annu Rev Microbiol (2006) 60:281–303. 10.1146/annurev.micro.59.030804.121055 [DOI] [PubMed] [Google Scholar]
- 43. Izacc SM, Gomez FJ, Jesuino RS, Fonseca CA, Felipe MS, Deepe GS, et al. Molecular Cloning, Characterization and Expression of the Heat Shock Protein 60 Gene From the Human Pathogenic Fungus Paracoccidioides Brasiliensis. Med Mycol (2001) 39:445–55. 10.1080/mmy.39.5.445.455 [DOI] [PubMed] [Google Scholar]
- 44. Gomez FJ, Allendoerfer R, Deepe GS., Jr. Vaccination With Recombinant Heat Shock Protein 60 From Histoplasma Capsulatum Protects Mice Against Pulmonary Histoplasmosis. Infect Immun (1995) 63:2587–95. 10.1128/IAI.63.7.2587-2595.1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Cunha DA, Zancope-Oliveira RM, Sueli M, Felipe S, Salem-Izacc SM, Deepe GS, Jr., et al. Heterologous Expression, Purification, and Immunological Reactivity of a Recombinant HSP60 From Paracoccidioides Brasiliensis. Clin Diagn Lab Immunol (2002) 9:374–7. 10.1128/cdli.9.2.374-377.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Diez S, Gomez BL, Restrepo A, Hay RJ, Hamilton AJ. Paracoccidioides Brasiliensis 87-Kilodalton Antigen, a Heat Shock Protein Useful in Diagnosis: Characterization, Purification, and Detection in Biopsy Material Via Immunohistochemistry. J Clin Microbiol (2002) 40:359–65. 10.1128/jcm.40.2.359-365.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bisio LC, Silva SP, Pereira IS, Xavier MA, Venancio EJ, Puccia R, et al. A New Paracoccidioides Brasiliensis 70-kDa Heat Shock Protein Reacts With Sera From Paracoccidioidomycosis Patients. Med Mycol (2005) 43:495–503. 10.1080/13693780400029478 [DOI] [PubMed] [Google Scholar]
- 48. de Castro LF, Ferreira MC, da Silva RM, Blotta MH, Longhi LN, Mamoni RL. Characterization of the Immune Response in Human Paracoccidioidomycosis. J Infect (2013) 67:470–85. 10.1016/j.jinf.2013.07.019 [DOI] [PubMed] [Google Scholar]
- 49. Souto JT, Figueiredo F, Furlanetto A, Pfeffer K, Rossi MA, Silva JS. Interferon-Gamma and Tumor Necrosis Factor-Alpha Determine Resistance to Paracoccidioides Brasiliensis Infection in Mice. Am J Pathol (2000) 156:1811–20. 10.1016/s0002-9440(10)65053-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Tristao FSM, Rocha FA, Carlos D, Ketelut-Carneiro N, Souza COS, Milanezi CM, et al. Th17-Inducing Cytokines IL-6 and IL-23 are Crucial for Granuloma Formation During Experimental Paracoccidioidomycosis. Front Immunol (2017) 8:949. 10.3389/fimmu.2017.00949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kursar M, Koch M, Mittrucker HW, Nouailles G, Bonhagen K, Kamradt T, et al. Cutting Edge: Regulatory T Cells Prevent Efficient Clearance of Mycobacterium Tuberculosis. J Immunol (2007) 178:2661–5. 10.4049/jimmunol.178.5.2661 [DOI] [PubMed] [Google Scholar]
- 52. Li MO, Wan YY, Flavell RAT. Cell-Produced Transforming Growth Factor-Beta1 Controls T Cell Tolerance and Regulates Th1- and Th17-cell Differentiation. Immunity (2007) 26:579–91. 10.1016/j.immuni.2007.03.014 [DOI] [PubMed] [Google Scholar]
- 53. Calich VL, Vaz CA, Burger E. Immunity to Paracoccidioides Brasiliensis Infection. Res Immunol (1998) 149:407–17; discussion 499-500. 10.1016/s0923-2494(98)80764-5 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://www.uniprot.org/, A0A0A2V091, https://www.uniprot.org/, C1GPI5, https://www.uniprot.org/, C1HDS5, https://www.uniprot.org/, C1H2V9, https://www.uniprot.org/, A0A0A2V0A9, https://www.uniprot.org/, C1GP19, https://www.uniprot.org/, C1GXU1, https://www.uniprot.org/, C1GRW7, https://www.uniprot.org/, C1H2K5, https://www.uniprot.org/, C1GNW1, https://www.uniprot.org/, C1H8U1, https://www.uniprot.org/, C1GRA7, https://www.uniprot.org/, C1GWA6, https://www.uniprot.org/, C1GWJ4.