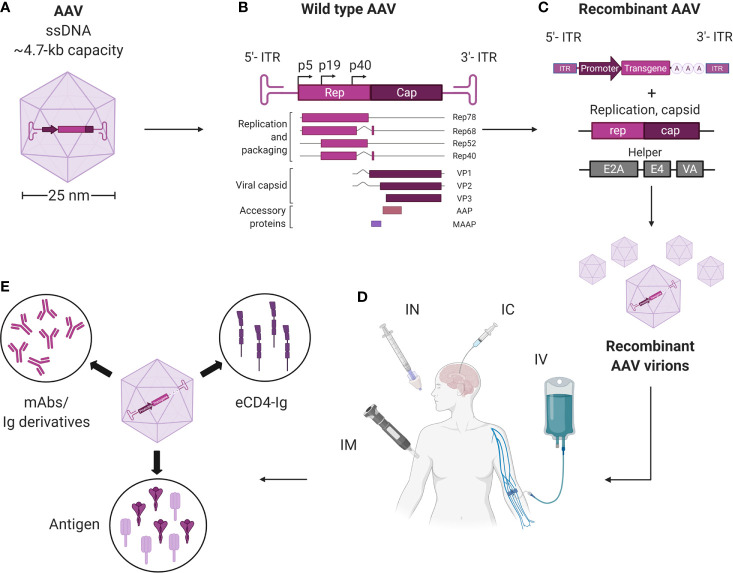
Figure 1.
Overview of AAV vectors harnessed for vectored immunoprophylaxis and therapeutics. (A) AAVs are small (~25 nm), non-enveloped viruses and have a 4.7-kb, single-stranded, linear DNA (ssDNA) genome encoding four open reading frames. (B) rep encodes the four genes required for genome replication (Rep78, Rep68, Rep52, and Rep40) and cap encodes the structural proteins of the viral capsid (VP1, VP2 and VP3). A third gene, which encodes assembly activating protein (AAP), is embedded within the cap coding sequence in a different reading frame and has been shown to promote virion assembly. The fourth ORF encodes for the recently discovered membrane associated accessory protein (MAAP). The role of MAAP is yet to be clearly defined. (C) Providing rep and cap in trans enables a transgene of interest to be packaged inside the capsid to generate a replication-incompetent vector (recombinant AAV; rAAV). (D) rAAVs may be delivered via intramuscnular (IM), intranasal (IN), intracerebral (IC), and intravenous (IV) routes. (E) rAAVs expressing mAbs, eCD4-Ig and pathogenic antigens can be administered via different routes for therapeutics and immunization against infectious diseases. Created with BioRender.com.