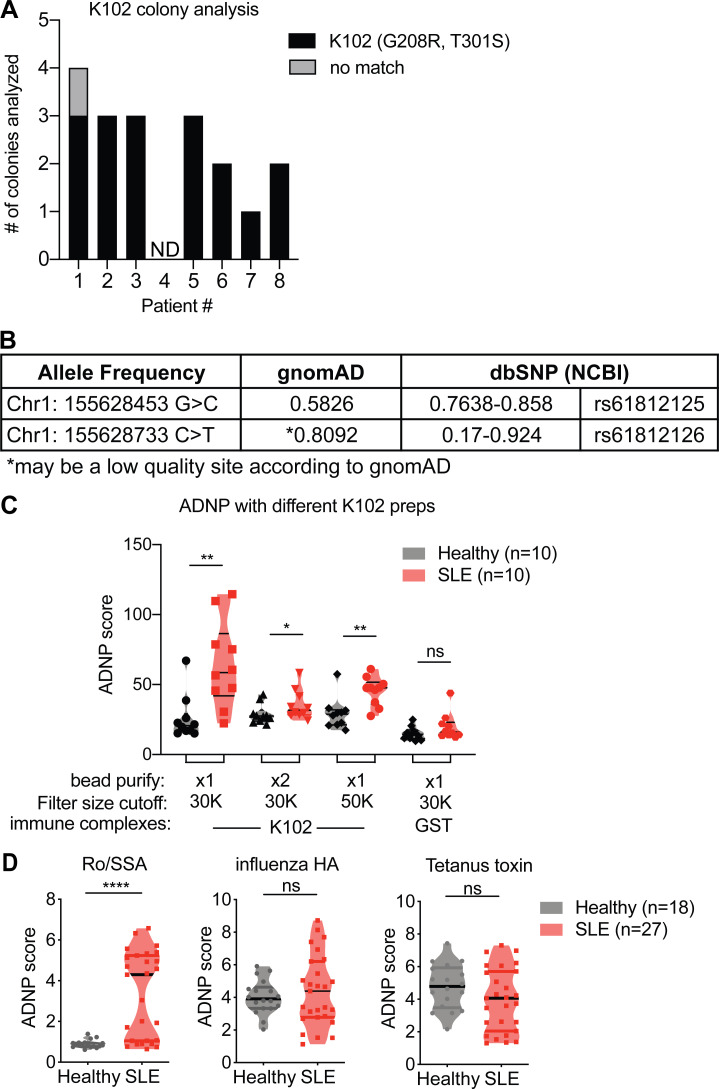
Figure S4.
ERV-K102 cDNA sequence analysis and ADNP with different K102 protein preps and other antigens. (A) Sequencing analysis of ERV-K102 DNA amplified from SLE PBMC cDNA and cloned into a sequencing vector. BLAT results against for each of the inserts against hg38 per SLE patient (n = 8). No match, no alignment between insert and hg38. (B) Summary of the allele frequency for Chr1: 155628453 G>C (T301S) and Chr1: 155628733 C>T (G208R). (C) ADNP assay with immune complexes containing recombinant ERV-K SU envelope protein that were purified through an additional round of GST-bead purification or larger cutoff spin columns. Purified GST was used as a negative control. (D) ADNP assay with immune complexes containing the indicated antigens and healthy or SLE plasma. Mann–Whitney t test was performed to calculate statistical significance. *, P < 0.05; **, P < 0.01; ****, P < 0.0001.